Copper Chloride Reaction With Sodium Hydroxide . The reaction of hexaaquacopper (ii) ions with hydroxide ions. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide precipitates copper(ii) hydroxide: This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. Use either a net ionic equations. You can use ammonia solution instead of sodium hydroxide. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Electrolysis of sodium chloride solution produces results at each electrode.
from www.youtube.com
Electrolysis of sodium chloride solution produces results at each electrode. This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. You can use ammonia solution instead of sodium hydroxide. Use either a net ionic equations. The reaction of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Sodium hydroxide precipitates copper(ii) hydroxide: 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution.
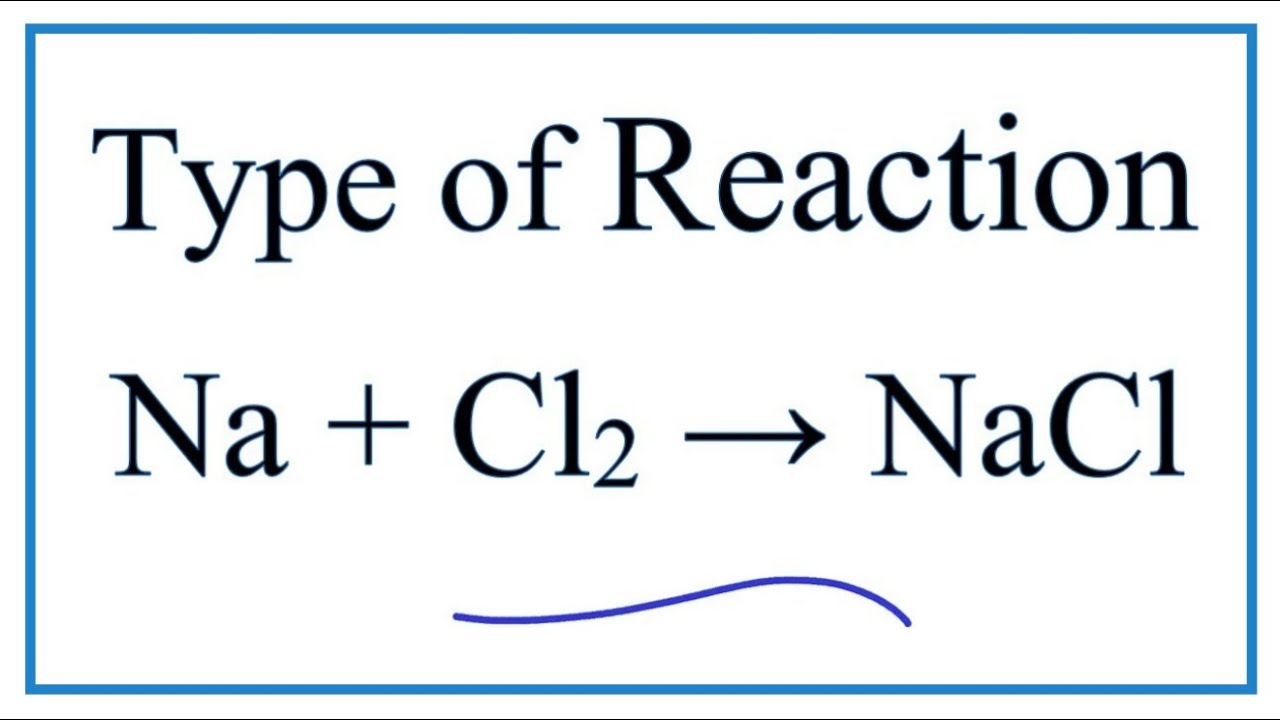
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube
Copper Chloride Reaction With Sodium Hydroxide A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. The reaction of hexaaquacopper (ii) ions with hydroxide ions. Use either a net ionic equations. Sodium hydroxide precipitates copper(ii) hydroxide: You can use ammonia solution instead of sodium hydroxide. This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Electrolysis of sodium chloride solution produces results at each electrode. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory.
From stock.adobe.com
Double displacement reaction sodium hydroxide and copper sulfate Copper Chloride Reaction With Sodium Hydroxide The reaction of hexaaquacopper (ii) ions with hydroxide ions. You can use ammonia solution instead of sodium hydroxide. Sodium hydroxide precipitates copper(ii) hydroxide: Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Electrolysis of. Copper Chloride Reaction With Sodium Hydroxide.
From www.youtube.com
Sodium hydroxide and ammonia added to copper (II) chloride solution Copper Chloride Reaction With Sodium Hydroxide Use either a net ionic equations. This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The reaction of hexaaquacopper (ii) ions with hydroxide ions. Electrolysis of sodium chloride solution produces results at each. Copper Chloride Reaction With Sodium Hydroxide.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Copper Chloride Reaction With Sodium Hydroxide You can use ammonia solution instead of sodium hydroxide. Sodium hydroxide precipitates copper(ii) hydroxide: 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Use either a net ionic equations. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Yes, the reaction. Copper Chloride Reaction With Sodium Hydroxide.
From spmscience.blog.onlinetuition.com.my
Electrolysis of Copper (II) Chloride Solution SPM Science Copper Chloride Reaction With Sodium Hydroxide Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. The reaction of hexaaquacopper (ii) ions with hydroxide ions. Use either a net ionic equations. Electrolysis of sodium chloride solution produces results at each. Copper Chloride Reaction With Sodium Hydroxide.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write Copper Chloride Reaction With Sodium Hydroxide This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. The reaction of hexaaquacopper (ii) ions with hydroxide ions. Electrolysis of sodium chloride solution produces results at each electrode. Use either a net. Copper Chloride Reaction With Sodium Hydroxide.
From solvedlib.com
Write net ionic equations for the reaction, if any, t… SolvedLib Copper Chloride Reaction With Sodium Hydroxide The reaction of hexaaquacopper (ii) ions with hydroxide ions. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Use either a net ionic equations. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Electrolysis of sodium chloride solution produces results at each electrode.. Copper Chloride Reaction With Sodium Hydroxide.
From www.meritnation.com
What is formed when CuSO4 reacts with Sodium hydroxide solution Also Copper Chloride Reaction With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Use either a net ionic equations. The reaction of hexaaquacopper (ii) ions with hydroxide ions. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. A reaction of copper (ii) chloride with sodium hydroxide to. Copper Chloride Reaction With Sodium Hydroxide.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube Copper Chloride Reaction With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Electrolysis of sodium chloride solution produces results. Copper Chloride Reaction With Sodium Hydroxide.
From proper-cooking.info
Sodium Hydroxide Skeletal Structure Copper Chloride Reaction With Sodium Hydroxide Electrolysis of sodium chloride solution produces results at each electrode. You can use ammonia solution instead of sodium hydroxide. Sodium hydroxide precipitates copper(ii) hydroxide: Use either a net ionic equations. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. Yes, the reaction is correct however it may be preferable to either. Copper Chloride Reaction With Sodium Hydroxide.
From studylib.net
Analytical Chemistry (Use of NaOH and NH4OH) Copper Chloride Reaction With Sodium Hydroxide Sodium hydroxide precipitates copper(ii) hydroxide: The reaction of hexaaquacopper (ii) ions with hydroxide ions. You can use ammonia solution instead of sodium hydroxide. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Yes, the reaction is correct however it may be preferable to either include all or no spectator. Copper Chloride Reaction With Sodium Hydroxide.
From ar.inspiredpencil.com
Copper Ii Chloride Lewis Structure Copper Chloride Reaction With Sodium Hydroxide Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Electrolysis of sodium chloride solution produces results at each electrode. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Sodium hydroxide precipitates copper(ii) hydroxide: Hydroxide ions (from, say, sodium hydroxide solution). Copper Chloride Reaction With Sodium Hydroxide.
From express.adobe.com
Zinc and Copper Chloride Reaction Copper Chloride Reaction With Sodium Hydroxide This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide precipitates copper(ii) hydroxide: The reaction of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions. Copper Chloride Reaction With Sodium Hydroxide.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Copper Chloride Reaction With Sodium Hydroxide A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Sodium hydroxide precipitates copper(ii) hydroxide: Electrolysis of sodium chloride solution produces results at each electrode. Use either a net ionic equations. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. Yes,. Copper Chloride Reaction With Sodium Hydroxide.
From www.alamy.com
Sodium Hydroxide and Sodium Chloride Molecular Model of Atom. Vector Copper Chloride Reaction With Sodium Hydroxide A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Sodium hydroxide precipitates copper(ii) hydroxide: Electrolysis of sodium chloride solution produces results at each electrode. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. 7 rows solutions containing copper (ii) ions. Copper Chloride Reaction With Sodium Hydroxide.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Copper Chloride Reaction With Sodium Hydroxide This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. The reaction of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the. Copper Chloride Reaction With Sodium Hydroxide.
From courses.lumenlearning.com
Electrolysis Chemistry Copper Chloride Reaction With Sodium Hydroxide You can use ammonia solution instead of sodium hydroxide. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. The reaction of hexaaquacopper (ii) ions with hydroxide ions. Electrolysis of sodium chloride solution produces results at each electrode. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with. Copper Chloride Reaction With Sodium Hydroxide.
From fineartamerica.com
Precipitate Of Copper (ii) Chloride In Sodium Hydroxide Photograph by Copper Chloride Reaction With Sodium Hydroxide Electrolysis of sodium chloride solution produces results at each electrode. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached. Copper Chloride Reaction With Sodium Hydroxide.
From www.youtube.com
Reaction between calcium chloride and sodium hydroxide Copper Chloride Reaction With Sodium Hydroxide Use either a net ionic equations. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. The reaction of hexaaquacopper (ii) ions with hydroxide ions. 7 rows solutions containing copper (ii) ions form a. Copper Chloride Reaction With Sodium Hydroxide.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Copper Chloride Reaction With Sodium Hydroxide This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. The reaction of hexaaquacopper (ii) ions with hydroxide ions. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached. Copper Chloride Reaction With Sodium Hydroxide.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Copper Chloride Reaction With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. Electrolysis of sodium chloride solution produces results at each electrode. Yes, the reaction is correct however it may be preferable to either include all or. Copper Chloride Reaction With Sodium Hydroxide.
From www.alamy.com
Ii Hydroxide High Resolution Stock Photography and Images Alamy Copper Chloride Reaction With Sodium Hydroxide A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. You can use ammonia solution instead of sodium hydroxide. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. Yes, the reaction is correct however it may be preferable to either include. Copper Chloride Reaction With Sodium Hydroxide.
From hxetahfoe.blob.core.windows.net
Copper Chloride Reaction With Iron at Pauline Estill blog Copper Chloride Reaction With Sodium Hydroxide A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. You can use ammonia solution. Copper Chloride Reaction With Sodium Hydroxide.
From brainly.in
What are the products of Copper sulphate + sodium hydroxide? Brainly.in Copper Chloride Reaction With Sodium Hydroxide The reaction of hexaaquacopper (ii) ions with hydroxide ions. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Electrolysis of sodium chloride solution produces results at each electrode. This reaction demonstrates. Copper Chloride Reaction With Sodium Hydroxide.
From spark.adobe.com
Zinc and Copper Chloride Reaction Copper Chloride Reaction With Sodium Hydroxide You can use ammonia solution instead of sodium hydroxide. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands. Copper Chloride Reaction With Sodium Hydroxide.
From www.slideserve.com
PPT Write down the formulae for Silver chloride Sodium hydroxide Copper Chloride Reaction With Sodium Hydroxide Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. You can use ammonia solution instead of sodium hydroxide. Use either a net ionic equations. Electrolysis of sodium chloride solution produces results at each electrode. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the. Copper Chloride Reaction With Sodium Hydroxide.
From mammothmemory.net
Sodium reaction with hydrochloric acid is violent and quick Copper Chloride Reaction With Sodium Hydroxide Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Use either a net ionic equations. Electrolysis of sodium chloride solution produces results at each electrode. Sodium hydroxide precipitates copper(ii) hydroxide: This reaction demonstrates. Copper Chloride Reaction With Sodium Hydroxide.
From gideonkruwboyd.blogspot.com
Ammonium Chloride and Sodium Hydroxide Net Ionic Equation GideonkruwBoyd Copper Chloride Reaction With Sodium Hydroxide Electrolysis of sodium chloride solution produces results at each electrode. This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water. Copper Chloride Reaction With Sodium Hydroxide.
From brainly.in
Copper sulphate reacts with sodium hydroxide to form blue precipitate Copper Chloride Reaction With Sodium Hydroxide The reaction of hexaaquacopper (ii) ions with hydroxide ions. Sodium hydroxide precipitates copper(ii) hydroxide: 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. This reaction demonstrates the difference of solubility of copper chloride in. Copper Chloride Reaction With Sodium Hydroxide.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Copper Chloride Reaction With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. Electrolysis of sodium chloride solution produces results at each electrode. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide. Copper Chloride Reaction With Sodium Hydroxide.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper Chloride Reaction With Sodium Hydroxide A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Sodium hydroxide precipitates copper(ii) hydroxide: This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. Electrolysis of sodium chloride solution produces results at each electrode. You can use ammonia solution instead. Copper Chloride Reaction With Sodium Hydroxide.
From gioplnhkl.blob.core.windows.net
Copper Ii Nitrate And Potassium Hydroxide at Ryan Sindelar blog Copper Chloride Reaction With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. Sodium hydroxide precipitates copper(ii). Copper Chloride Reaction With Sodium Hydroxide.
From insende.netlify.app
30+ Hydrochloric Acid And Sodium Hydroxide Equation Insende Copper Chloride Reaction With Sodium Hydroxide A reaction of copper (ii) chloride with sodium hydroxide to produce copper (ii) hydroxide precipitate is performed in the laboratory. Use either a net ionic equations. You can use ammonia solution instead of sodium hydroxide. Sodium hydroxide precipitates copper(ii) hydroxide: Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. This reaction. Copper Chloride Reaction With Sodium Hydroxide.
From www.reddit.com
Precipitate Colors of Metal Ions in Aqueous Ammonia and Sodium Copper Chloride Reaction With Sodium Hydroxide Sodium hydroxide precipitates copper(ii) hydroxide: This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. The reaction of hexaaquacopper (ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper. Electrolysis of sodium chloride solution produces results at each. Copper Chloride Reaction With Sodium Hydroxide.
From studylib.net
Laboratory 5 Stoichiometry of Copper (II) Chloride and Aluminum Copper Chloride Reaction With Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Use either a net ionic equations. Sodium hydroxide precipitates copper(ii) hydroxide: Yes, the reaction is correct however it may be preferable to either include all or no spectator ions. This reaction demonstrates the difference of solubility of copper chloride in water, and then. Copper Chloride Reaction With Sodium Hydroxide.
From www.youtube.com
Reaction of Copper Sulphate (CuSO4) with Sodium Hydroxide (NaOH) YouTube Copper Chloride Reaction With Sodium Hydroxide Sodium hydroxide precipitates copper(ii) hydroxide: You can use ammonia solution instead of sodium hydroxide. Use either a net ionic equations. The reaction of hexaaquacopper (ii) ions with hydroxide ions. This reaction demonstrates the difference of solubility of copper chloride in water, and then with addition of sodium hydroxide. 7 rows solutions containing copper (ii) ions form a blue precipitate when. Copper Chloride Reaction With Sodium Hydroxide.