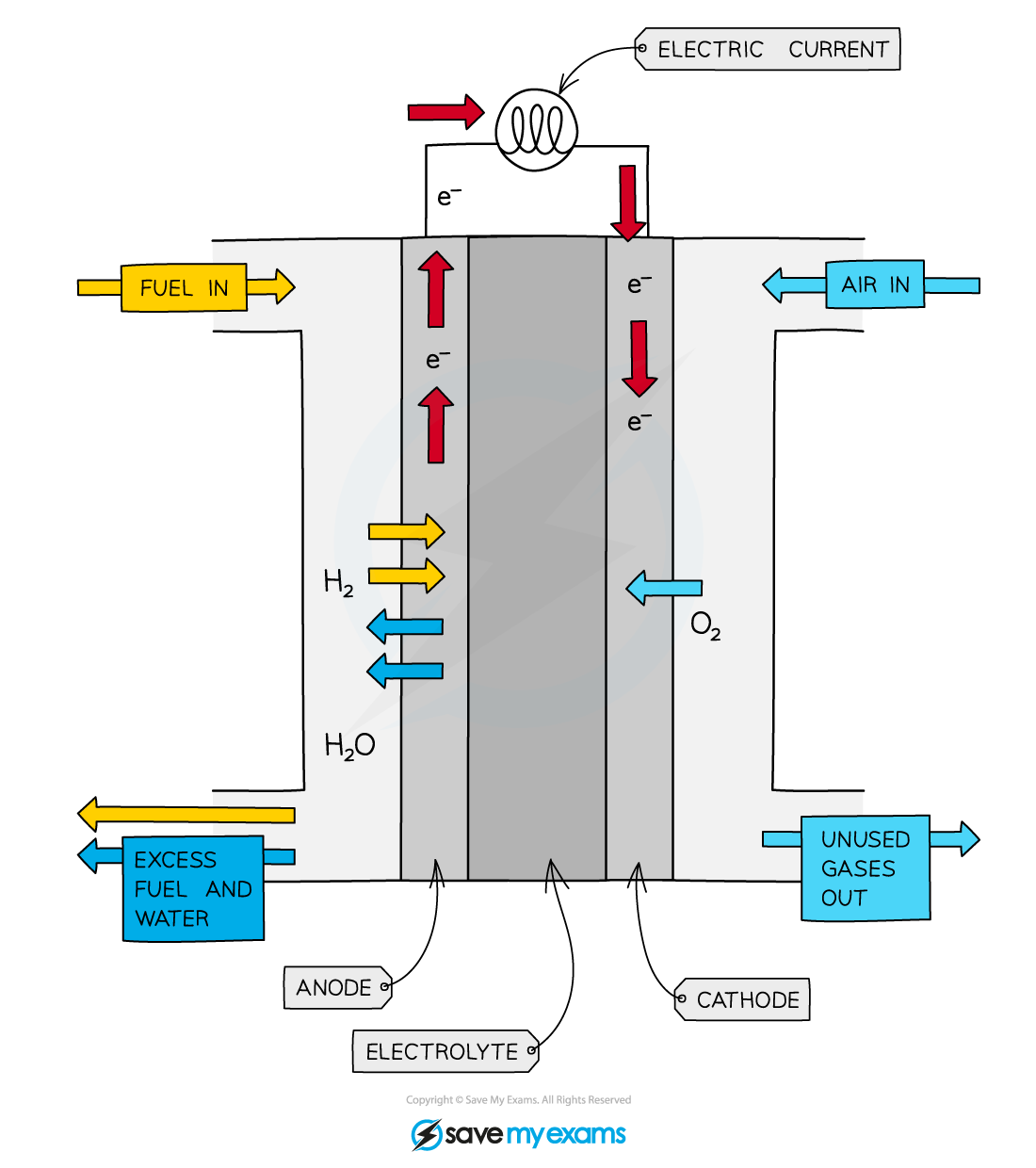
Hydrogen Fuel Cell Electrode Reactions . One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. Hydrogen used in fuel cells can be extracted. Oxygen is supplied to a similar. Hydrogen + oxygen → water. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. One fuel cell alone only produces a small amount of power. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The overall equation for the. A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte.
from www.linstitute.net
The overall equation for the. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen used in fuel cells can be extracted. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. One fuel cell alone only produces a small amount of power. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. Oxygen is supplied to a similar.
Edexcel A Level Chemistry复习笔记6.1.8 Fuel Cells翰林国际教育
Hydrogen Fuel Cell Electrode Reactions Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen + oxygen → water. A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. One fuel cell alone only produces a small amount of power. One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Oxygen is supplied to a similar. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. The overall equation for the. Hydrogen used in fuel cells can be extracted. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte.
From www.dreamstime.com
Hydrogen Electrode Stock Illustrations 81 Hydrogen Electrode Stock Hydrogen Fuel Cell Electrode Reactions Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. Hydrogen + oxygen → water. One fuel cell alone only produces a small amount of power. Hydrogen used in fuel cells can be extracted. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by. Hydrogen Fuel Cell Electrode Reactions.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians Hydrogen Fuel Cell Electrode Reactions Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. Hydrogen used. Hydrogen Fuel Cell Electrode Reactions.
From www.slideserve.com
PPT Fuel Cell Catalysts Based on Metal Nanoparticles PowerPoint Hydrogen Fuel Cell Electrode Reactions Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. Hydrogen used in fuel cells can be extracted. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. A fuel cell consists of two electrodes—a negative electrode (or anode) and. Hydrogen Fuel Cell Electrode Reactions.
From www.dreamstime.com
Hydrogen Fuel Cell Electrode Reactions Stock Photo Image of Hydrogen Fuel Cell Electrode Reactions Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. One type of fuel cell. Hydrogen Fuel Cell Electrode Reactions.
From www.chfca.ca
About Fuel Cells CHFCA Hydrogen Fuel Cell Electrode Reactions The overall equation for the. One fuel cell alone only produces a small amount of power. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A hydrogen fuel cell enables hydrogen to be combined. Hydrogen Fuel Cell Electrode Reactions.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Hydrogen Fuel Cell Electrode Reactions Hydrogen + oxygen → water. Hydrogen used in fuel cells can be extracted. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Oxygen is supplied to a similar. One type. Hydrogen Fuel Cell Electrode Reactions.
From www.cataler.co.jp
Electrode catalyst for fuel cell Automotive Engineering Exposition Hydrogen Fuel Cell Electrode Reactions The overall equation for the. Oxygen is supplied to a similar. Hydrogen + oxygen → water. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. Hydrogen enters the cell through a. Hydrogen Fuel Cell Electrode Reactions.
From www.mdpi.com
Energies Free FullText Developments in Hydrogen Fuel Cells Hydrogen Fuel Cell Electrode Reactions The overall equation for the. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. Hydrogen Fuel Cell Electrode Reactions.
From china.denora.com
Anodes and Cathodes for Fuel Cells De Nora Hydrogen Fuel Cell Electrode Reactions Oxygen is supplied to a similar. Hydrogen + oxygen → water. Hydrogen used in fuel cells can be extracted. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at.. Hydrogen Fuel Cell Electrode Reactions.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Hydrogen Fuel Cell Electrode Reactions Hydrogen used in fuel cells can be extracted. The overall equation for the. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar. A hydrogen fuel cell enables. Hydrogen Fuel Cell Electrode Reactions.
From www.mdpi.com
Processes Free FullText CFD Modeling and Experimental Validation Hydrogen Fuel Cell Electrode Reactions One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. Hydrogen used in fuel cells can be extracted. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen + oxygen → water. The overall equation for the. Oxygen is supplied to a similar. A fuel cell. Hydrogen Fuel Cell Electrode Reactions.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Hydrogen Fuel Cell Electrode Reactions Oxygen is supplied to a similar. Hydrogen + oxygen → water. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. The overall equation for the. Fuel cells this type of electrochemical. Hydrogen Fuel Cell Electrode Reactions.
From ar.inspiredpencil.com
How Hydrogen Fuel Cells Work Hydrogen Fuel Cell Electrode Reactions Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. One fuel cell alone only produces a small amount of power. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. The overall equation for the. A fuel cell consists of two electrodes—a negative electrode (or. Hydrogen Fuel Cell Electrode Reactions.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars Hydrogen Fuel Cell Electrode Reactions Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. Oxygen is supplied to a similar. Hydrogen + oxygen → water. One fuel cell alone only produces a small amount of power. Revision. Hydrogen Fuel Cell Electrode Reactions.
From www.dreamstime.com
Hydrogen Electrode Stock Illustrations 79 Hydrogen Electrode Stock Hydrogen Fuel Cell Electrode Reactions Hydrogen used in fuel cells can be extracted. Hydrogen + oxygen → water. A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. The overall equation for the. Hydrogen enters the cell through. Hydrogen Fuel Cell Electrode Reactions.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Hydrogen Fuel Cell Electrode Reactions A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. Hydrogen used in fuel cells can be extracted. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written. Hydrogen Fuel Cell Electrode Reactions.
From www.mdpi.com
Catalysts Free FullText Fundamentals of Gas Diffusion Electrodes Hydrogen Fuel Cell Electrode Reactions Hydrogen + oxygen → water. Hydrogen used in fuel cells can be extracted. Oxygen is supplied to a similar. One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. One fuel cell alone only produces a small amount of power. Fuel cells this type of electrochemical cell is used to generate an. Hydrogen Fuel Cell Electrode Reactions.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Hydrogen Fuel Cell Electrode Reactions One fuel cell alone only produces a small amount of power. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen used in fuel cells can be extracted. The overall equation for the. A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. Hydrogen + oxygen. Hydrogen Fuel Cell Electrode Reactions.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Hydrogen Fuel Cell Electrode Reactions A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. One fuel cell alone only produces a small amount of power. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. The overall equation for the. Hydrogen + oxygen → water. Hydrogen used. Hydrogen Fuel Cell Electrode Reactions.
From www.alamy.com
Fuel cell diagram. Vector. Device that converts chemical potential Hydrogen Fuel Cell Electrode Reactions A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. The overall equation for the. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. Hydrogen + oxygen → water. One fuel cell alone only produces a small amount of power.. Hydrogen Fuel Cell Electrode Reactions.
From quizlet.com
HydrogenOxygen Fuel Cell Diagram Quizlet Hydrogen Fuel Cell Electrode Reactions Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen used in fuel cells can be extracted. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and. Hydrogen Fuel Cell Electrode Reactions.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记6.1.8 Fuel Cells翰林国际教育 Hydrogen Fuel Cell Electrode Reactions Hydrogen used in fuel cells can be extracted. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. One type of fuel cell contains hydrogen that reacts with oxygen. Hydrogen Fuel Cell Electrode Reactions.
From mavink.com
Hydrogen Production Schematic Hydrogen Fuel Cell Electrode Reactions Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. The overall equation for the. One type of fuel cell contains hydrogen that reacts with oxygen from the air to. Hydrogen Fuel Cell Electrode Reactions.
From chemistry.stackexchange.com
electrochemistry Electrode polarity in fuel cells Chemistry Stack Hydrogen Fuel Cell Electrode Reactions One fuel cell alone only produces a small amount of power. Hydrogen + oxygen → water. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. Hydrogen used in fuel cells can be. Hydrogen Fuel Cell Electrode Reactions.
From large.stanford.edu
Hydrogen Fuel Cells Hydrogen Fuel Cell Electrode Reactions Hydrogen + oxygen → water. Hydrogen used in fuel cells can be extracted. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. One fuel cell alone only produces a small amount of power. The overall equation for the. Revision notes on electrode reactions in hydrogen fuel cells for the aqa. Hydrogen Fuel Cell Electrode Reactions.
From revisechemistry.uk
Organic chemistry (chemistry only) OCR Gateway C6 revisechemistry.uk Hydrogen Fuel Cell Electrode Reactions Hydrogen used in fuel cells can be extracted. Oxygen is supplied to a similar. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. Revision notes on electrode reactions in hydrogen fuel cells. Hydrogen Fuel Cell Electrode Reactions.
From www.slideserve.com
PPT Hydrogen electrolysis PowerPoint Presentation, free download ID Hydrogen Fuel Cell Electrode Reactions Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. The overall equation for the. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged.. Hydrogen Fuel Cell Electrode Reactions.
From saylordotorg.github.io
Electrochemistry Hydrogen Fuel Cell Electrode Reactions One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. One fuel. Hydrogen Fuel Cell Electrode Reactions.
From www.lapsedphysicist.org
Hydrogen and Fuel Cells Important Parts of Our Energy Future Hydrogen Fuel Cell Electrode Reactions A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. One fuel cell alone only produces a small amount of power. Hydrogen + oxygen → water. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. A hydrogen fuel cell enables. Hydrogen Fuel Cell Electrode Reactions.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Hydrogen Fuel Cell Electrode Reactions Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. Hydrogen +. Hydrogen Fuel Cell Electrode Reactions.
From gioqaodmb.blob.core.windows.net
Electrodes In Hydrogen Fuel Cell at Martha Briggs blog Hydrogen Fuel Cell Electrode Reactions A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. One fuel. Hydrogen Fuel Cell Electrode Reactions.
From fixcarbattery.blogspot.com
Recondition Car battery Magnesium copper water battery Hydrogen Fuel Cell Electrode Reactions A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Hydrogen. Hydrogen Fuel Cell Electrode Reactions.
From saylordotorg.github.io
Electrochemistry Hydrogen Fuel Cell Electrode Reactions Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Oxygen is supplied to a similar. Hydrogen used in fuel cells can be extracted. Fuel cells this type of. Hydrogen Fuel Cell Electrode Reactions.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Hydrogen Fuel Cell Electrode Reactions Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte.. Hydrogen Fuel Cell Electrode Reactions.
From www.researchgate.net
Scheme of twoelectrode system for overall water splitting. HER Hydrogen Fuel Cell Electrode Reactions Fuel cells this type of electrochemical cell is used to generate an electrical current without needing to be recharged. One type of fuel cell contains hydrogen that reacts with oxygen from the air to produce a voltage. One fuel cell alone only produces a small amount of power. A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen. Hydrogen Fuel Cell Electrode Reactions.