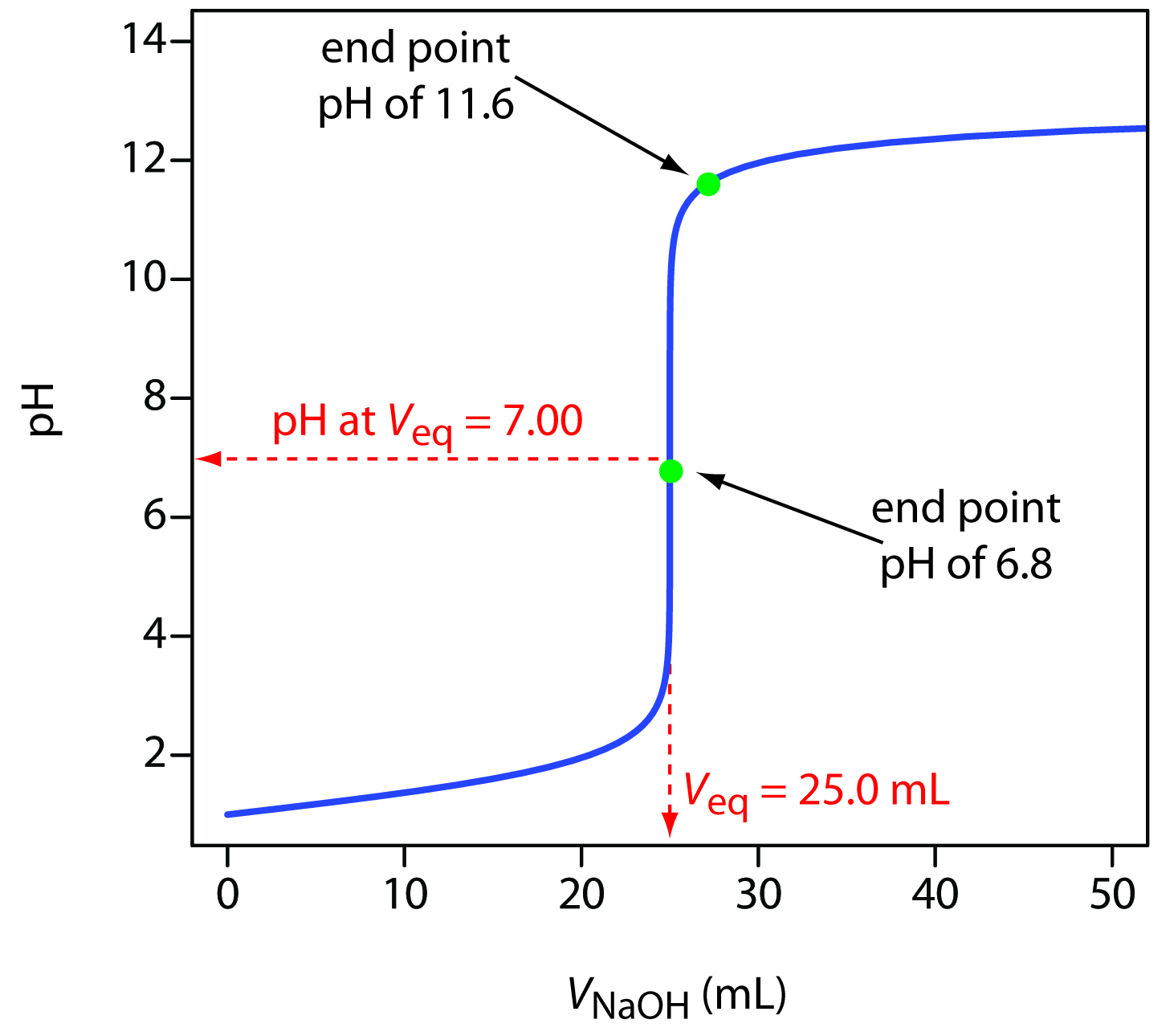
Titration Curve Of A Strong Acid . For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. For example, titrating sodium hydroxide against ethanoic. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. Titration curves for strong acid v strong base. An example of such a titration would be acetic acid (ch 3 cooh). All acid titration curves follow the. A graph showing the change in ph during a titration is called a titration curve.
from chem.libretexts.org
Titration curves for strong acid v strong base. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. An example of such a titration would be acetic acid (ch 3 cooh). All acid titration curves follow the. A graph showing the change in ph during a titration is called a titration curve. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. For example, titrating sodium hydroxide against ethanoic. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve.
9.1 Overview of Titrimetry Chemistry LibreTexts
Titration Curve Of A Strong Acid If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. A graph showing the change in ph during a titration is called a titration curve. All acid titration curves follow the. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Titration curves for strong acid v strong base. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. For example, titrating sodium hydroxide against ethanoic. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. An example of such a titration would be acetic acid (ch 3 cooh).
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Titration Curve Of A Strong Acid For example, titrating sodium hydroxide against ethanoic. All acid titration curves follow the. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. A graph showing the change in ph during a titration is called a titration curve. Titration curves for strong acid v strong base. If the ph of an acid solution. Titration Curve Of A Strong Acid.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Curve Of A Strong Acid Titration curves for strong acid v strong base. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. A graph showing the change in ph. Titration Curve Of A Strong Acid.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration Curve Of A Strong Acid Titration curves for strong acid v strong base. A graph showing the change in ph during a titration is called a titration curve. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. For example, titrating sodium hydroxide against ethanoic. If the ph of an acid solution is plotted against the amount of. Titration Curve Of A Strong Acid.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve Titration Curve Of A Strong Acid All acid titration curves follow the. A graph showing the change in ph during a titration is called a titration curve. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Titration curves for strong acid v. Titration Curve Of A Strong Acid.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Curve Of A Strong Acid For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. For example, titrating sodium hydroxide against ethanoic. A graph showing. Titration Curve Of A Strong Acid.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Curve Of A Strong Acid If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. Titration Curve Of A Strong Acid.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Titration Curve Of A Strong Acid For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Titration curves for strong acid v strong base. A graph showing the change in ph during a titration is called a titration curve. If the ph of. Titration Curve Of A Strong Acid.
From mungfali.com
Strong Acid And Base Titration Curve Titration Curve Of A Strong Acid Titration curves for strong acid v strong base. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. All acid titration curves follow the. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. For. Titration Curve Of A Strong Acid.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Of A Strong Acid We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. For example, titrating sodium hydroxide against ethanoic. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Titration curves for. Titration Curve Of A Strong Acid.
From brainly.com
The graphs labeled (a) and (b) show the titration curves for two equal Titration Curve Of A Strong Acid If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. A graph showing the change in ph during a titration is called a titration curve. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base.. Titration Curve Of A Strong Acid.
From www.chegg.com
Solved The titration curve for a weak acid/strong base Titration Curve Of A Strong Acid An example of such a titration would be acetic acid (ch 3 cooh). A graph showing the change in ph during a titration is called a titration curve. For example, titrating sodium hydroxide against ethanoic. Titration curves for strong acid v strong base. If the ph of an acid solution is plotted against the amount of base added during a. Titration Curve Of A Strong Acid.
From www.vrogue.co
Strong Acid And Base Titration Curve vrogue.co Titration Curve Of A Strong Acid An example of such a titration would be acetic acid (ch 3 cooh). We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. All acid titration curves follow the. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is. Titration Curve Of A Strong Acid.
From slidetodoc.com
Acid Base Titrations Titration Curve A titration curve Titration Curve Of A Strong Acid For example, titrating sodium hydroxide against ethanoic. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape. Titration Curve Of A Strong Acid.
From mungfali.com
Strong Acid And Base Titration Curve Titration Curve Of A Strong Acid An example of such a titration would be acetic acid (ch 3 cooh). We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. All acid. Titration Curve Of A Strong Acid.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Titration Curve Of A Strong Acid Titration curves for strong acid v strong base. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. All acid titration curves follow the. For. Titration Curve Of A Strong Acid.
From mavink.com
Strong Acid And Base Titration Curve Titration Curve Of A Strong Acid A graph showing the change in ph during a titration is called a titration curve. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. An example of such a titration would be acetic acid (ch 3. Titration Curve Of A Strong Acid.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Of A Strong Acid Titration curves for strong acid v strong base. A graph showing the change in ph during a titration is called a titration curve. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. If the ph of. Titration Curve Of A Strong Acid.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Titration Curve Of A Strong Acid A graph showing the change in ph during a titration is called a titration curve. An example of such a titration would be acetic acid (ch 3 cooh). For example, titrating sodium hydroxide against ethanoic. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. All acid titration curves follow the. Titration curves. Titration Curve Of A Strong Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Of A Strong Acid A graph showing the change in ph during a titration is called a titration curve. An example of such a titration would be acetic acid (ch 3 cooh). All acid titration curves follow the. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. For the titration of a strong acid with a. Titration Curve Of A Strong Acid.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Of A Strong Acid Titration curves for strong acid v strong base. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on. Titration Curve Of A Strong Acid.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Curve Of A Strong Acid A graph showing the change in ph during a titration is called a titration curve. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. An example of such a titration would be acetic acid (ch 3. Titration Curve Of A Strong Acid.
From www.doubtnut.com
The principle on conductometric titration is based in the fact that Titration Curve Of A Strong Acid We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. Titration curves for strong acid v strong base. All acid. Titration Curve Of A Strong Acid.
From mavink.com
Monoprotic Acid Titration Curve Titration Curve Of A Strong Acid Titration curves for strong acid v strong base. A graph showing the change in ph during a titration is called a titration curve. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. For the titration of a strong acid with a. Titration Curve Of A Strong Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Of A Strong Acid We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. A graph showing the change in ph during a titration is called a titration curve. An example of such a titration would be acetic acid (ch 3 cooh). If the ph of an acid solution is plotted against the amount of base added. Titration Curve Of A Strong Acid.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare Titration Curve Of A Strong Acid For example, titrating sodium hydroxide against ethanoic. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. All acid titration curves follow the. A graph showing the change in ph during a titration is called a titration curve. An example of such. Titration Curve Of A Strong Acid.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Titration Curve Of A Strong Acid If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. An example of such a titration would be acetic acid (ch 3 cooh). For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of. Titration Curve Of A Strong Acid.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Of A Strong Acid An example of such a titration would be acetic acid (ch 3 cooh). We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. For example, titrating sodium hydroxide against ethanoic. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points. Titration Curve Of A Strong Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Of A Strong Acid Titration curves for strong acid v strong base. A graph showing the change in ph during a titration is called a titration curve. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. We'll take hydrochloric acid. Titration Curve Of A Strong Acid.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curve Of A Strong Acid For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution stoichiometry. If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration. Titration Curve Of A Strong Acid.
From morioh.com
Acid Base Titration Curves PH Calculations Titration Curve Of A Strong Acid We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. For example, titrating sodium hydroxide against ethanoic. An example of such a titration would be acetic acid (ch 3 cooh). Titration curves for strong acid v strong base. For the titration of a strong acid with a strong base, the equivalence point occurs. Titration Curve Of A Strong Acid.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Of A Strong Acid All acid titration curves follow the. Titration curves for strong acid v strong base. An example of such a titration would be acetic acid (ch 3 cooh). We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. A graph showing the change in ph during a titration is called a titration curve. If. Titration Curve Of A Strong Acid.
From studylib.net
Titration Curve weak base with strong acid START Titration Curve Of A Strong Acid We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base. All acid titration curves follow the. An example of such a titration would be acetic acid (ch 3 cooh). For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on. Titration Curve Of A Strong Acid.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Of A Strong Acid An example of such a titration would be acetic acid (ch 3 cooh). A graph showing the change in ph during a titration is called a titration curve. For example, titrating sodium hydroxide against ethanoic. All acid titration curves follow the. For the titration of a strong acid with a strong base, the equivalence point occurs at a ph of. Titration Curve Of A Strong Acid.
From mavink.com
Strong Acid And Base Titration Curve Titration Curve Of A Strong Acid If the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. A graph showing the change in ph during a titration is called a titration curve. We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base.. Titration Curve Of A Strong Acid.
From mungfali.com
Strong Acid And Base Titration Curve Titration Curve Of A Strong Acid For example, titrating sodium hydroxide against ethanoic. A graph showing the change in ph during a titration is called a titration curve. An example of such a titration would be acetic acid (ch 3 cooh). All acid titration curves follow the. If the ph of an acid solution is plotted against the amount of base added during a titration, the. Titration Curve Of A Strong Acid.