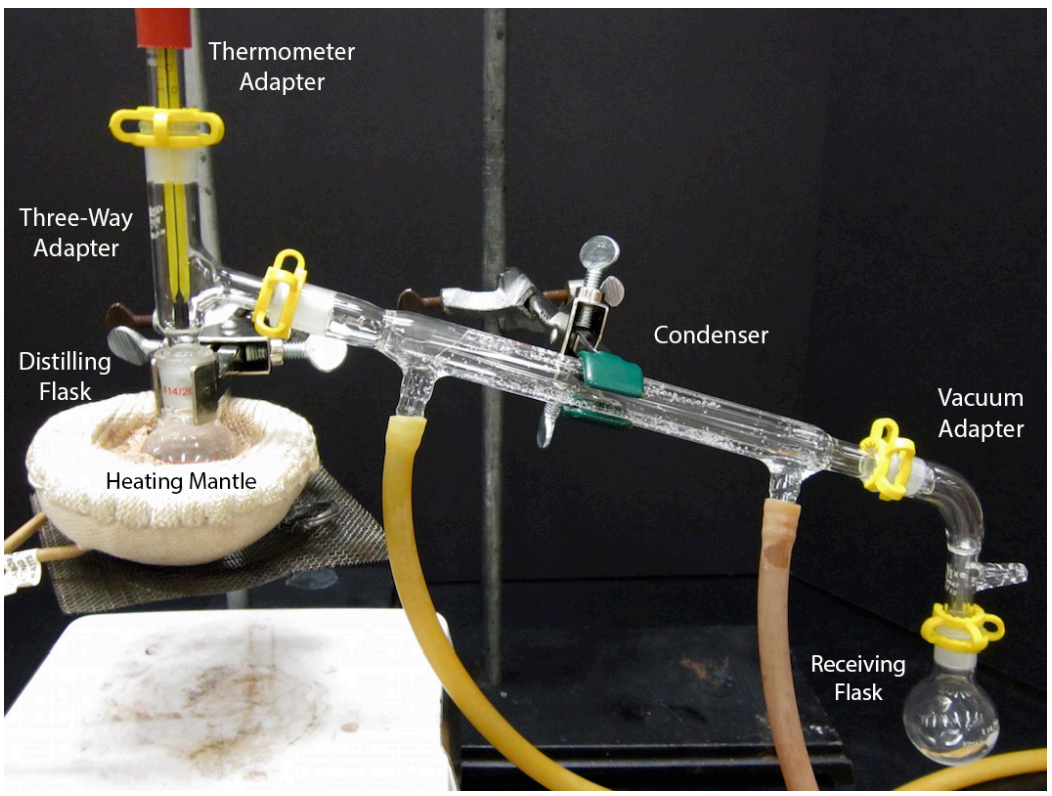
Vacuum Distillation Method . Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. In order to boil these compounds, heating to high temperatures is an inefficient method. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or.
from chem.libretexts.org
In order to boil these compounds, heating to high temperatures is an inefficient method. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup.
5.2C StepbyStep Procedures Chemistry LibreTexts
Vacuum Distillation Method In order to boil these compounds, heating to high temperatures is an inefficient method. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. In order to boil these compounds, heating to high temperatures is an inefficient method.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Vacuum Distillation Method A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In order to boil these compounds, heating to high temperatures is an inefficient method. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Vacuum. Vacuum Distillation Method.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Vacuum Distillation Method Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In order to boil these compounds, heating. Vacuum Distillation Method.
From supertekglassware.com
Vacuum Distillation Vacuum Distillation Method Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. In order to boil these compounds, heating to high temperatures is an inefficient method. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with. Vacuum Distillation Method.
From www.pressurecontrolsolutions.com
Vacuum Distillation issues? Call Pressure Control Solutions! Vacuum Distillation Method In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. A vacuum distillation apparatus is shown in figure 5.50, using. Vacuum Distillation Method.
From www.vecteezy.com
Distillation process diagram for education 3227893 Vector Art at Vecteezy Vacuum Distillation Method Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In order to boil these compounds, heating to high temperatures. Vacuum Distillation Method.
From www.labrotovap.com
The Vacuum Distillation Methods for Cannabis Extraction Process Lab Vacuum Distillation Method Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is ideal for separating mixtures. Vacuum Distillation Method.
From www.researchgate.net
The schematic for the vacuum distillation process. Download Vacuum Distillation Method Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with. Vacuum Distillation Method.
From www.gustawater.com
The Ultimate Guide to Distillation and Distillation Columns Vacuum Distillation Method In order to boil these compounds, heating to high temperatures is an inefficient method. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with. Vacuum Distillation Method.
From cartoondealer.com
Fractional Distillation Of Crude Oil Diagram Vector Illustration Vacuum Distillation Method In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point. Vacuum Distillation Method.
From www.alamy.com
Vacuum Distillation System Stock Vector Image & Art Alamy Vacuum Distillation Method Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is a technique for the purification or separation of liquids or solvents with. Vacuum Distillation Method.
From www.scienceabc.com
Denatured Alcohol Definition, Properties, Examples And Uses Vacuum Distillation Method A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Vacuum. Vacuum Distillation Method.
From ar.inspiredpencil.com
Fractional Distillation Apparatus Vacuum Distillation Method A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum. Vacuum Distillation Method.
From foodtechnotes.com
Distillation Principle and Types Food Tech Notes Vacuum Distillation Method A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In order to boil these compounds, heating. Vacuum Distillation Method.
From www.researchgate.net
Schematic representation of the multistage vacuum membrane distillation Vacuum Distillation Method In order to boil these compounds, heating to high temperatures is an inefficient method. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum. Vacuum Distillation Method.
From www.vecteezy.com
Diagram showing Distillation Separating Mixtures 2896364 Vector Art at Vacuum Distillation Method Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation apparatus is shown in figure 5.50, using. Vacuum Distillation Method.
From www.thoughtco.com
What Is Distillation? Principles and Uses Vacuum Distillation Method Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In order to boil these compounds, heating. Vacuum Distillation Method.
From mavink.com
Distillation Phase Diagram Vacuum Distillation Method Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with. Vacuum Distillation Method.
From www.researchgate.net
The vacuum distillation apparatus scheme. Download Scientific Diagram Vacuum Distillation Method Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in. Vacuum Distillation Method.
From www.e-education.psu.edu
Atmospheric and Vacuum Distillation Units FSC 432 Petroleum Refining Vacuum Distillation Method Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or. Vacuum Distillation Method.
From www.youtube.com
Vacuum Distillation YouTube Vacuum Distillation Method In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is ideal for separating mixtures. Vacuum Distillation Method.
From thepetrosolutions.com
Crude Oil Distillation Unit Vacuum Distillation Method Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In order to boil these compounds, heating. Vacuum Distillation Method.
From www.researchgate.net
Diagram of the vacuum distillation still used for purification Vacuum Distillation Method In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or. Vacuum Distillation Method.
From www.geeksforgeeks.org
Methods of Purification of Organic Compounds Vacuum Distillation Method Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. A vacuum distillation apparatus is shown in figure 5.50, using. Vacuum Distillation Method.
From chem.libretexts.org
5.5D StepbyStep Procedures for Steam Distillation Chemistry LibreTexts Vacuum Distillation Method In order to boil these compounds, heating to high temperatures is an inefficient method. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with. Vacuum Distillation Method.
From www.electronicpro.co.za
1000mL 24/29 Glass Vacuum Distillation Extraction Distilling Apparatus Vacuum Distillation Method Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In order to boil these compounds, heating to high temperatures is an inefficient method. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or. Vacuum Distillation Method.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Vacuum Distillation Method A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. Vacuum distillation is ideal for separating mixtures of liquids with. Vacuum Distillation Method.
From oldmymages.blogspot.com
Vacuum Distillation Column P&id Oldmymages Vacuum Distillation Method Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. In order to boil these compounds, heating. Vacuum Distillation Method.
From ar.inspiredpencil.com
Distillation Diagram Vacuum Distillation Method Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. In order to boil these compounds, heating to high temperatures is an inefficient method. A vacuum distillation apparatus is shown in figure 5.50, using. Vacuum Distillation Method.
From www.bioxtralabs.com
Distillation Equipment Wiped Film and Short Path Vacuum Distillation Method Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum. Vacuum Distillation Method.
From www.coursehero.com
[Solved] what is vacuum distillation method? what is difference between Vacuum Distillation Method Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with. Vacuum Distillation Method.
From www.vecteezy.com
Distillation, distillation under reduced pressure, vacuum distillation Vacuum Distillation Method A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with. Vacuum Distillation Method.
From www.britannica.com
distillation summary Britannica Vacuum Distillation Method In order to boil these compounds, heating to high temperatures is an inefficient method. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum. Vacuum Distillation Method.
From www.shemmassianconsulting.com
Reactions and Separations for the MCAT Everything You Need to Know Vacuum Distillation Method Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Boiling commences when the vapor pressure of a liquid or. Vacuum Distillation Method.
From glossary.periodni.com
Chemistry Glossary Search results for 'distillation' Vacuum Distillation Method A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. Boiling commences when the vapor pressure of a liquid or. Vacuum Distillation Method.
From chem.libretexts.org
5.2C StepbyStep Procedures Chemistry LibreTexts Vacuum Distillation Method A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. In order to boil these compounds, heating to high temperatures is an inefficient method. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. Boiling commences when the vapor pressure of a liquid or. Vacuum Distillation Method.