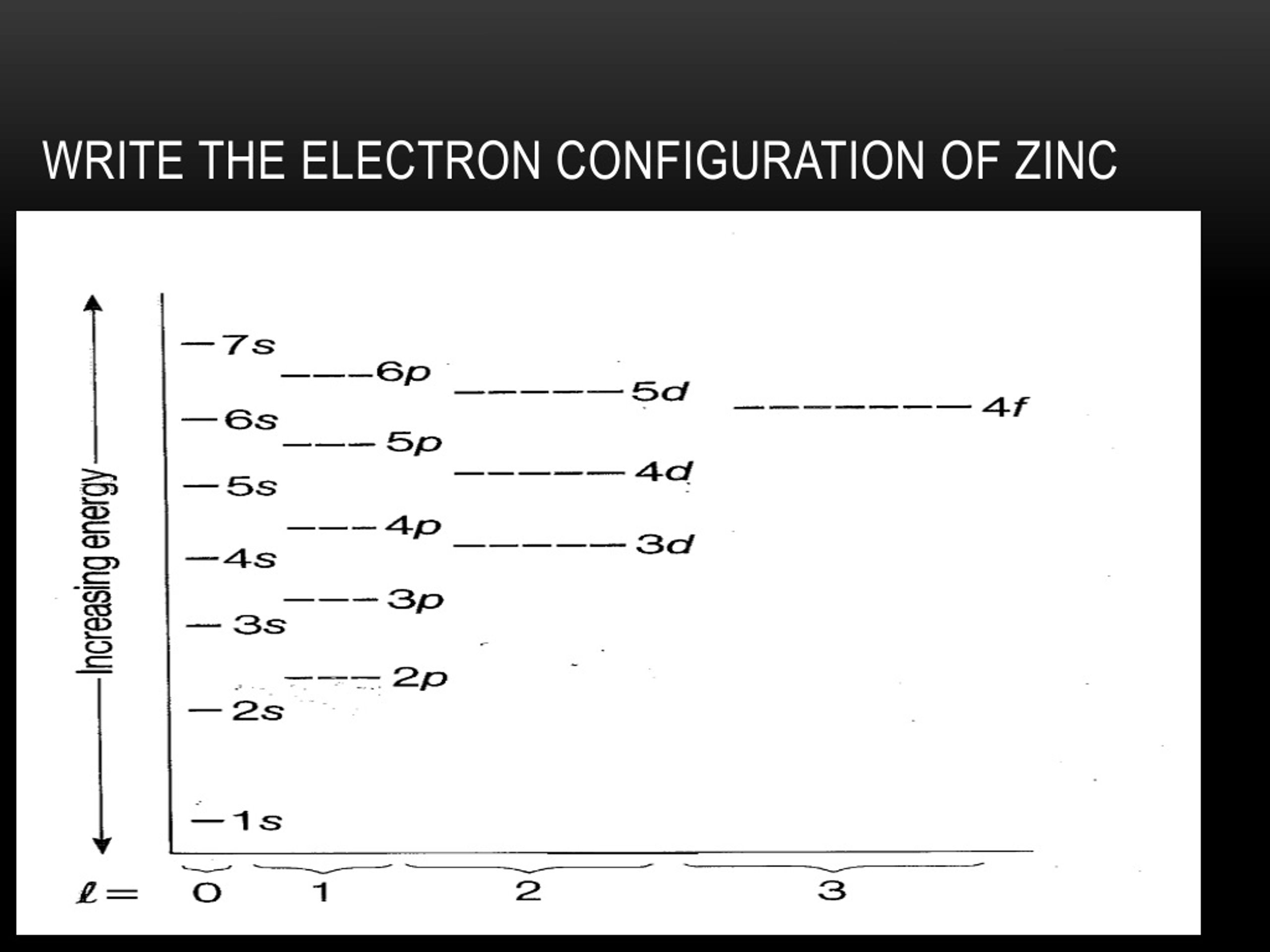
Zinc Valence Electron Configuration . to find the number of valence electrons for zinc (zn) we need to look at its. the precise and accurate valency of zinc is +2. Zinc is the metal transition element and hence it belongs to the d block category. find the full electronic configuration and valence electrons of any periodic element using this electron. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. So, zinc gains stability after losing out the 2 electrons. so, the valence electrons of zinc are 2.
from www.slideserve.com
find the full electronic configuration and valence electrons of any periodic element using this electron. the precise and accurate valency of zinc is +2. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. Zinc is the metal transition element and hence it belongs to the d block category. so, the valence electrons of zinc are 2. to find the number of valence electrons for zinc (zn) we need to look at its. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. So, zinc gains stability after losing out the 2 electrons.
PPT Chapter 7 “Ionic and Metallic Bonding” PowerPoint Presentation
Zinc Valence Electron Configuration Zinc is the metal transition element and hence it belongs to the d block category. the precise and accurate valency of zinc is +2. Zinc is the metal transition element and hence it belongs to the d block category. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. So, zinc gains stability after losing out the 2 electrons. find the full electronic configuration and valence electrons of any periodic element using this electron. to find the number of valence electrons for zinc (zn) we need to look at its. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. so, the valence electrons of zinc are 2.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Zinc Valence Electron Configuration The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. So, zinc gains stability after losing out the 2 electrons. so, the valence electrons of zinc are 2. to find the number of valence electrons for zinc (zn) we need to look at its. the valence orbital diagram for zn clearly illustrates the. Zinc Valence Electron Configuration.
From material-properties.org
Zinc Protons Neutrons Electrons Electron Configuration Zinc Valence Electron Configuration So, zinc gains stability after losing out the 2 electrons. find the full electronic configuration and valence electrons of any periodic element using this electron. the precise and accurate valency of zinc is +2. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. Zinc is. Zinc Valence Electron Configuration.
From animalia-life.club
Zinc Electron Configuration Zinc Valence Electron Configuration So, zinc gains stability after losing out the 2 electrons. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. Zinc is the metal transition element and hence it belongs to the d block category. the precise and accurate valency of zinc is +2. find the. Zinc Valence Electron Configuration.
From dxouakext.blob.core.windows.net
Zinc Ion Noble Gas Configuration at Jason Thomas blog Zinc Valence Electron Configuration the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. find the full electronic configuration and valence electrons of any periodic element using this electron. so, the valence electrons of zinc are 2. to find the number of valence electrons for zinc (zn) we need. Zinc Valence Electron Configuration.
From ar.inspiredpencil.com
Zinc Electron Configuration Zinc Valence Electron Configuration so, the valence electrons of zinc are 2. So, zinc gains stability after losing out the 2 electrons. find the full electronic configuration and valence electrons of any periodic element using this electron. the precise and accurate valency of zinc is +2. to find the number of valence electrons for zinc (zn) we need to look. Zinc Valence Electron Configuration.
From valenceelectrons.com
How to Find the Valence Electrons for Zinc (Zn)? Zinc Valence Electron Configuration The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. to find the number of valence electrons for zinc (zn) we need to look at its. Zinc is the metal transition element and hence it belongs to the d block category. the precise and accurate valency of zinc is +2. so, the valence. Zinc Valence Electron Configuration.
From www.youtube.com
Electron Configuration for Zn and Zn2+ (Zinc and Zinc ion) YouTube Zinc Valence Electron Configuration So, zinc gains stability after losing out the 2 electrons. so, the valence electrons of zinc are 2. find the full electronic configuration and valence electrons of any periodic element using this electron. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. the precise and accurate valency of zinc is +2. Zinc. Zinc Valence Electron Configuration.
From www.youtube.com
How to find Protons & Electrons for the Zn 2+ (Zinc ion) YouTube Zinc Valence Electron Configuration find the full electronic configuration and valence electrons of any periodic element using this electron. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. so, the valence electrons of zinc are 2. Zinc is the metal transition element and hence it belongs to the d. Zinc Valence Electron Configuration.
From dxoohxnae.blob.core.windows.net
Zinc Electron Config at Randy Justice blog Zinc Valence Electron Configuration find the full electronic configuration and valence electrons of any periodic element using this electron. So, zinc gains stability after losing out the 2 electrons. so, the valence electrons of zinc are 2. Zinc is the metal transition element and hence it belongs to the d block category. the valence orbital diagram for zn clearly illustrates the. Zinc Valence Electron Configuration.
From animalia-life.club
Zinc Electron Configuration Zinc Valence Electron Configuration find the full electronic configuration and valence electrons of any periodic element using this electron. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. to find the number of valence electrons for zinc (zn) we need to look at its. So, zinc gains stability after. Zinc Valence Electron Configuration.
From animalia-life.club
Zinc Electron Configuration Zinc Valence Electron Configuration to find the number of valence electrons for zinc (zn) we need to look at its. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. the precise and accurate valency of zinc is +2. So, zinc gains stability after losing out the 2 electrons. The. Zinc Valence Electron Configuration.
From www.sciencephoto.com
Zinc, atomic structure Stock Image C013/1553 Science Photo Library Zinc Valence Electron Configuration to find the number of valence electrons for zinc (zn) we need to look at its. So, zinc gains stability after losing out the 2 electrons. find the full electronic configuration and valence electrons of any periodic element using this electron. the precise and accurate valency of zinc is +2. The outermost shell of zinc contains 2. Zinc Valence Electron Configuration.
From dxonyecim.blob.core.windows.net
Zn Electron Configuration Diagram at Beatrice Fitch blog Zinc Valence Electron Configuration so, the valence electrons of zinc are 2. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. Zinc is the metal transition element and hence it belongs to the d block category. So, zinc gains stability after losing out the 2 electrons. to find the. Zinc Valence Electron Configuration.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Zinc Valence Electron Configuration So, zinc gains stability after losing out the 2 electrons. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. the precise and accurate valency of zinc is +2. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. so, the valence. Zinc Valence Electron Configuration.
From www.adda247.com
What is the valency of zinc? Zinc Valence Electron Configuration the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. So, zinc gains stability after losing out the 2 electrons. to find the number of valence electrons for zinc (zn) we need to. Zinc Valence Electron Configuration.
From www.sciencephoto.com
Zinc electron configuration Stock Image C029/5029 Science Photo Zinc Valence Electron Configuration The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. so, the valence electrons of zinc are 2. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. find the full electronic configuration and valence electrons of any periodic element using this. Zinc Valence Electron Configuration.
From animalia-life.club
Zinc Electron Configuration Zinc Valence Electron Configuration Zinc is the metal transition element and hence it belongs to the d block category. So, zinc gains stability after losing out the 2 electrons. so, the valence electrons of zinc are 2. to find the number of valence electrons for zinc (zn) we need to look at its. the valence orbital diagram for zn clearly illustrates. Zinc Valence Electron Configuration.
From www.youtube.com
Electron Configuration of Zinc Zn Lesson YouTube Zinc Valence Electron Configuration the precise and accurate valency of zinc is +2. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. So, zinc gains stability after losing out the 2 electrons. to find the number of valence electrons for zinc (zn) we need to look at its. . Zinc Valence Electron Configuration.
From www.slideserve.com
PPT Chapter 7 “Ionic and Metallic Bonding” PowerPoint Presentation Zinc Valence Electron Configuration So, zinc gains stability after losing out the 2 electrons. the precise and accurate valency of zinc is +2. Zinc is the metal transition element and hence it belongs to the d block category. to find the number of valence electrons for zinc (zn) we need to look at its. The outermost shell of zinc contains 2 electrons. Zinc Valence Electron Configuration.
From dxonyecim.blob.core.windows.net
Zn Electron Configuration Diagram at Beatrice Fitch blog Zinc Valence Electron Configuration Zinc is the metal transition element and hence it belongs to the d block category. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. So, zinc gains stability after losing out the 2 electrons. the precise and accurate valency of zinc is +2. so, the. Zinc Valence Electron Configuration.
From www.livescience.com
Facts About Zinc Live Science Zinc Valence Electron Configuration Zinc is the metal transition element and hence it belongs to the d block category. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. to find the number of valence electrons for zinc (zn) we need to look at its. So, zinc gains stability after losing out the 2 electrons. so, the valence. Zinc Valence Electron Configuration.
From material-properties.org
Zinc Protons Neutrons Electrons Electron Configuration Zinc Valence Electron Configuration to find the number of valence electrons for zinc (zn) we need to look at its. so, the valence electrons of zinc are 2. So, zinc gains stability after losing out the 2 electrons. Zinc is the metal transition element and hence it belongs to the d block category. find the full electronic configuration and valence electrons. Zinc Valence Electron Configuration.
From www.alamy.com
Zinc (Zn). Diagram of the nuclear composition and electron Zinc Valence Electron Configuration the precise and accurate valency of zinc is +2. Zinc is the metal transition element and hence it belongs to the d block category. find the full electronic configuration and valence electrons of any periodic element using this electron. to find the number of valence electrons for zinc (zn) we need to look at its. So, zinc. Zinc Valence Electron Configuration.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence Zinc Valence Electron Configuration so, the valence electrons of zinc are 2. Zinc is the metal transition element and hence it belongs to the d block category. find the full electronic configuration and valence electrons of any periodic element using this electron. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. to find the number of. Zinc Valence Electron Configuration.
From animalia-life.club
Zinc Electron Configuration Zinc Valence Electron Configuration The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. so, the valence electrons of zinc are 2. So, zinc gains stability after losing out the 2 electrons. find the full electronic configuration and valence electrons of any periodic element using this electron. Zinc is the metal transition element and hence it belongs to. Zinc Valence Electron Configuration.
From animalia-life.club
Zinc Electron Configuration Zinc Valence Electron Configuration the precise and accurate valency of zinc is +2. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. to find the number of valence electrons for zinc (zn) we need to look at its. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying. Zinc Valence Electron Configuration.
From animalia-life.club
Zinc Electron Configuration Zinc Valence Electron Configuration So, zinc gains stability after losing out the 2 electrons. so, the valence electrons of zinc are 2. to find the number of valence electrons for zinc (zn) we need to look at its. find the full electronic configuration and valence electrons of any periodic element using this electron. Zinc is the metal transition element and hence. Zinc Valence Electron Configuration.
From www.youtube.com
zinc electronic configuration How to Write Zinc electronic Zinc Valence Electron Configuration the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. Zinc is the metal transition element and hence it belongs to the d block category. find the full electronic configuration and valence electrons of any periodic element using this electron. the precise and accurate valency of. Zinc Valence Electron Configuration.
From www.nuclear-power.com
Zinc Electron Affinity Electronegativity Ionization Energy of Zinc Valence Electron Configuration So, zinc gains stability after losing out the 2 electrons. to find the number of valence electrons for zinc (zn) we need to look at its. find the full electronic configuration and valence electrons of any periodic element using this electron. Zinc is the metal transition element and hence it belongs to the d block category. the. Zinc Valence Electron Configuration.
From www.alamy.com
Symbol and electron diagram for Zinc Stock Vector Image & Art Alamy Zinc Valence Electron Configuration the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying the 4s orbital. to find the number of valence electrons for zinc (zn) we need to look at its. the precise and accurate valency of zinc is +2. find the full electronic configuration and valence electrons of any. Zinc Valence Electron Configuration.
From pnghut.com
Electron Configuration Shell Atom Chemical Element Atomic Orbital Zinc Valence Electron Configuration Zinc is the metal transition element and hence it belongs to the d block category. So, zinc gains stability after losing out the 2 electrons. to find the number of valence electrons for zinc (zn) we need to look at its. find the full electronic configuration and valence electrons of any periodic element using this electron. the. Zinc Valence Electron Configuration.
From animalia-life.club
Zinc Electron Configuration Zinc Valence Electron Configuration So, zinc gains stability after losing out the 2 electrons. find the full electronic configuration and valence electrons of any periodic element using this electron. so, the valence electrons of zinc are 2. to find the number of valence electrons for zinc (zn) we need to look at its. the valence orbital diagram for zn clearly. Zinc Valence Electron Configuration.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Zinc Valence Electron Configuration so, the valence electrons of zinc are 2. to find the number of valence electrons for zinc (zn) we need to look at its. Zinc is the metal transition element and hence it belongs to the d block category. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying. Zinc Valence Electron Configuration.
From artoflifestyles.blogspot.com
Electron Configuration For Zn And Zn2+ (Zinc And Zinc Ion) YouTube Zinc Valence Electron Configuration So, zinc gains stability after losing out the 2 electrons. to find the number of valence electrons for zinc (zn) we need to look at its. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. the valence orbital diagram for zn clearly illustrates the electron configuration of zinc, with two valence electrons occupying. Zinc Valence Electron Configuration.
From www.alamy.com
Zinc (Zn). Diagram of the nuclear composition, electron configuration Zinc Valence Electron Configuration Zinc is the metal transition element and hence it belongs to the d block category. find the full electronic configuration and valence electrons of any periodic element using this electron. So, zinc gains stability after losing out the 2 electrons. to find the number of valence electrons for zinc (zn) we need to look at its. so,. Zinc Valence Electron Configuration.