Indicator For Citric Acid . In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. It is the purpose of this experiment to determine the. Because the indicator’s acid and base forms have different. Citric acid and sodium hydroxide are used for all three presented titrations. Refer to part 1 for the background information and the titration method. 3 naoh (aq) + h 3 c 6 h 5 o 7. Some citrus fruits taste more sour, and therefore are more acidic, than others. To determine the amount of citric acid present in fruit juices. During the first stages of the titration, the naoh will be completely neutralised, and an. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration.
from www.alamy.com
A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Because the indicator’s acid and base forms have different. To determine the amount of citric acid present in fruit juices. Refer to part 1 for the background information and the titration method. Citric acid and sodium hydroxide are used for all three presented titrations. 3 naoh (aq) + h 3 c 6 h 5 o 7. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. During the first stages of the titration, the naoh will be completely neutralised, and an. It is the purpose of this experiment to determine the. Some citrus fruits taste more sour, and therefore are more acidic, than others.
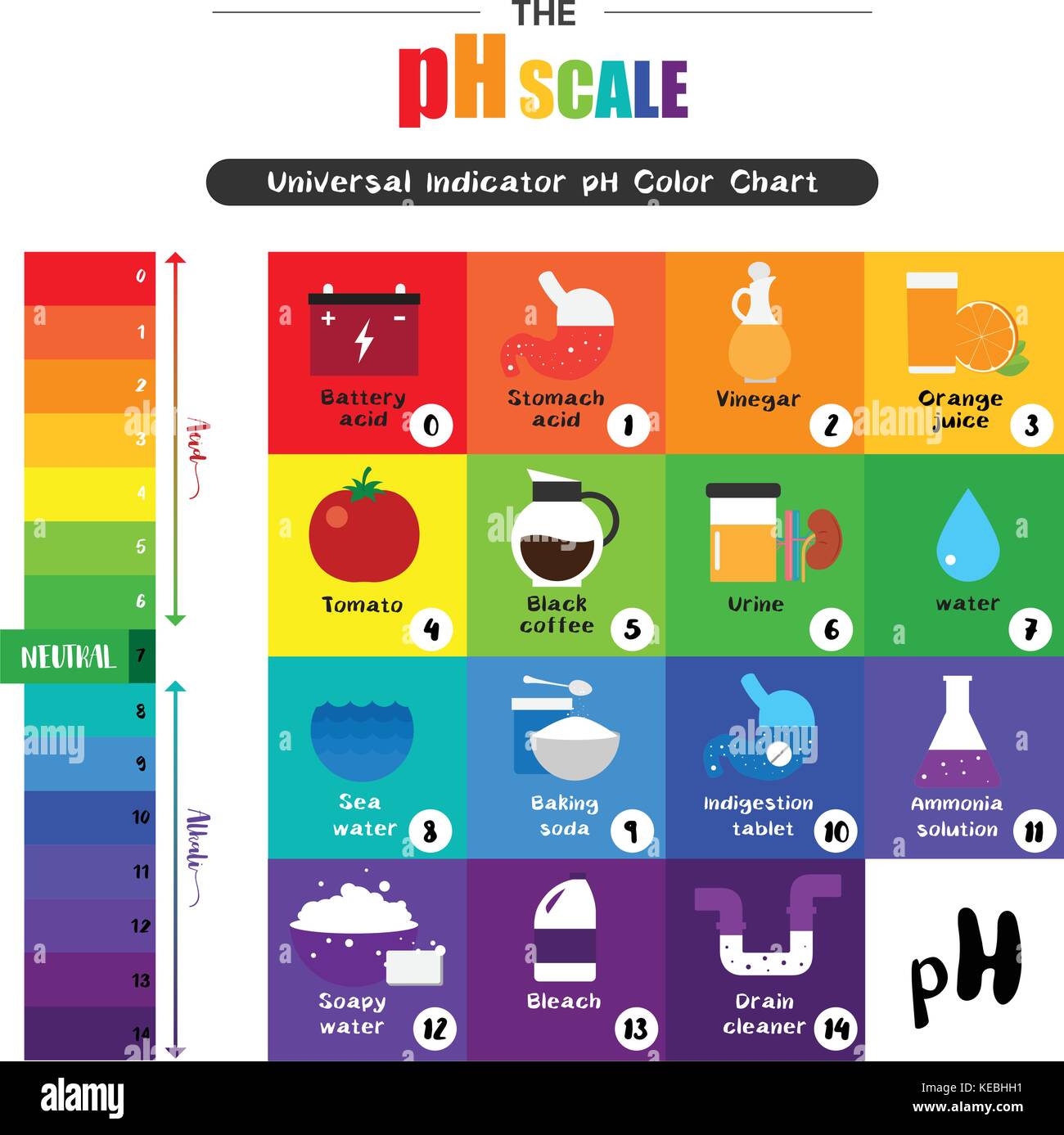
The pH scale Universal Indicator pH Color Chart diagram acidic Stock
Indicator For Citric Acid Refer to part 1 for the background information and the titration method. Because the indicator’s acid and base forms have different. Refer to part 1 for the background information and the titration method. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. During the first stages of the titration, the naoh will be completely neutralised, and an. Some citrus fruits taste more sour, and therefore are more acidic, than others. 3 naoh (aq) + h 3 c 6 h 5 o 7. It is the purpose of this experiment to determine the. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Citric acid and sodium hydroxide are used for all three presented titrations. To determine the amount of citric acid present in fruit juices.
From chemistrytalk.org
Citric Acid Cycle Explained ChemTalk Indicator For Citric Acid It is the purpose of this experiment to determine the. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Because the indicator’s acid and base forms have different. Citric acid and sodium hydroxide are used for all three presented titrations. To determine the amount of citric acid present in fruit juices. In the. Indicator For Citric Acid.
From www.studocu.com
Citric acid Estimation by Titration method ESTIMATION OF CITRIC ACID Indicator For Citric Acid Refer to part 1 for the background information and the titration method. To determine the amount of citric acid present in fruit juices. It is the purpose of this experiment to determine the. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Because the indicator’s acid and base forms have different. In the. Indicator For Citric Acid.
From www.youtube.com
Citric acid and Universal Indicator YouTube Indicator For Citric Acid Refer to part 1 for the background information and the titration method. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. Citric acid and sodium hydroxide are used for all three presented titrations. 3 naoh (aq) + h 3 c 6 h 5 o 7. A suitable. Indicator For Citric Acid.
From www.researchgate.net
Raman spectra of citric acid solid and its aqueous solution. Download Indicator For Citric Acid During the first stages of the titration, the naoh will be completely neutralised, and an. Because the indicator’s acid and base forms have different. To determine the amount of citric acid present in fruit juices. 3 naoh (aq) + h 3 c 6 h 5 o 7. It is the purpose of this experiment to determine the. A suitable indicator. Indicator For Citric Acid.
From www.alamy.com
The pH scale Universal Indicator pH Color Chart diagram acidic Stock Indicator For Citric Acid To determine the amount of citric acid present in fruit juices. Citric acid and sodium hydroxide are used for all three presented titrations. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. 3 naoh (aq) + h 3 c 6 h 5 o 7. Refer to part 1 for the background information and. Indicator For Citric Acid.
From www.studocu.com
5 Citric Acid Cycle Dr. Mosley's lecture + book notes from Biology Indicator For Citric Acid It is the purpose of this experiment to determine the. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. 3 naoh (aq) + h 3 c 6 h 5 o 7. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration.. Indicator For Citric Acid.
From stock.adobe.com
Citric acid cycle diagram, vector illustration molecular scheme Stock Indicator For Citric Acid During the first stages of the titration, the naoh will be completely neutralised, and an. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Some citrus fruits taste more sour, and therefore are more acidic, than others. It is the purpose of this experiment to determine the. 3 naoh (aq) + h 3. Indicator For Citric Acid.
From www.dreamstime.com
Citric acid stock illustration. Illustration of atoms 212807998 Indicator For Citric Acid Refer to part 1 for the background information and the titration method. 3 naoh (aq) + h 3 c 6 h 5 o 7. Some citrus fruits taste more sour, and therefore are more acidic, than others. Citric acid and sodium hydroxide are used for all three presented titrations. It is the purpose of this experiment to determine the. To. Indicator For Citric Acid.
From www.vectorstock.com
Chemical formula citric acid hand drawn diagram Vector Image Indicator For Citric Acid During the first stages of the titration, the naoh will be completely neutralised, and an. To determine the amount of citric acid present in fruit juices. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Citric acid and sodium hydroxide are used for all three presented titrations. Because the indicator’s acid and base. Indicator For Citric Acid.
From www.slideserve.com
PPT 2. The Citric Acid Cycle (CAC) PowerPoint Presentation, free Indicator For Citric Acid Citric acid and sodium hydroxide are used for all three presented titrations. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. 3 naoh (aq) + h 3 c 6 h 5 o 7. Refer to part 1 for the background information and the titration method. During the first stages of the titration, the. Indicator For Citric Acid.
From www.researchgate.net
1 H NMR spectra of (a) citric acid H 2 O pH = 3.5; (b) citric acid Indicator For Citric Acid In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. During the first stages of the titration, the naoh will be completely neutralised, and an. Citric acid and sodium hydroxide are used. Indicator For Citric Acid.
From www.sliderbase.com
Properties of Acids Bases Presentation Chemistry Indicator For Citric Acid Some citrus fruits taste more sour, and therefore are more acidic, than others. To determine the amount of citric acid present in fruit juices. It is the purpose of this experiment to determine the. Because the indicator’s acid and base forms have different. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Citric. Indicator For Citric Acid.
From quizlet.com
citric acid cycle Diagram Quizlet Indicator For Citric Acid 3 naoh (aq) + h 3 c 6 h 5 o 7. It is the purpose of this experiment to determine the. Because the indicator’s acid and base forms have different. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Refer to part 1 for the background information and the titration method. In. Indicator For Citric Acid.
From www.glam.com
What You Need To Know About Incorporating Citric Acid Into Your Indicator For Citric Acid 3 naoh (aq) + h 3 c 6 h 5 o 7. It is the purpose of this experiment to determine the. Some citrus fruits taste more sour, and therefore are more acidic, than others. To determine the amount of citric acid present in fruit juices. In the case of the citric acid titration, a known amount of orange juice. Indicator For Citric Acid.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Indicator For Citric Acid A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. During the first stages of the titration, the naoh will be completely neutralised, and an. Because the indicator’s acid and base forms have different. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask. Indicator For Citric Acid.
From www.dermaessentia.com
What is Citric acid ? Its sources and its benefits Derma Essentia Indicator For Citric Acid In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. Citric acid and sodium hydroxide are used for all three presented titrations. During the first stages of the titration, the naoh will be completely neutralised, and an. A suitable indicator has a colour change (endpoint) coinciding with the. Indicator For Citric Acid.
From www.researchgate.net
The citric acid cycle (4). Download Scientific Diagram Indicator For Citric Acid During the first stages of the titration, the naoh will be completely neutralised, and an. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. It is the purpose of this experiment to determine the. Because the indicator’s acid and base forms have different. Some citrus fruits taste. Indicator For Citric Acid.
From labmonk.com
Screening for citric acid producing organisms Labmonk Indicator For Citric Acid A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Refer to part 1 for the background information and the titration method. To determine the amount of citric acid present in fruit juices. Citric acid and sodium hydroxide are used for all three presented titrations. In the case of the citric acid titration, a. Indicator For Citric Acid.
From www.researchgate.net
FTIR spectra of citric acid (a), citric acidcoated MNPs (b), NH Indicator For Citric Acid Because the indicator’s acid and base forms have different. To determine the amount of citric acid present in fruit juices. 3 naoh (aq) + h 3 c 6 h 5 o 7. Citric acid and sodium hydroxide are used for all three presented titrations. Refer to part 1 for the background information and the titration method. During the first stages. Indicator For Citric Acid.
From hubpages.com
What is Universal Indicator and How To Use it Indicator For Citric Acid 3 naoh (aq) + h 3 c 6 h 5 o 7. To determine the amount of citric acid present in fruit juices. It is the purpose of this experiment to determine the. During the first stages of the titration, the naoh will be completely neutralised, and an. Refer to part 1 for the background information and the titration method.. Indicator For Citric Acid.
From labmonk.com
Screening for citric acid producing organisms Labmonk Indicator For Citric Acid A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Refer to part 1 for the background information and the titration method. Citric acid and sodium hydroxide are used for all three presented titrations. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask. Indicator For Citric Acid.
From draxe.com
What Is Citric Acid? Pros and Cons, Plus How to Use It Dr. Axe Indicator For Citric Acid A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. During the first stages of the titration, the naoh will be completely neutralised, and an. Because the indicator’s acid and base forms have different. Some citrus fruits taste more sour, and therefore are more acidic, than others. To determine the amount of citric acid. Indicator For Citric Acid.
From technologyinscience.blogspot.com.tr
BioResource Citric Acid Production by Aspergillus niger, Estimation Indicator For Citric Acid Citric acid and sodium hydroxide are used for all three presented titrations. 3 naoh (aq) + h 3 c 6 h 5 o 7. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Some citrus fruits taste more sour, and therefore are more acidic, than others. In the case of the citric acid. Indicator For Citric Acid.
From quizlet.com
Citric Acid Cycle Diagram Quizlet Indicator For Citric Acid A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. Refer to part 1 for the background information and the titration method. To determine the amount of citric acid present in fruit. Indicator For Citric Acid.
From www.drawittoknowit.com
Biochemistry Glossary Citric Acid Cycle Draw It to Know It Indicator For Citric Acid In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. It is the purpose of this experiment to determine the. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. To determine the amount of citric acid present in fruit juices. 3. Indicator For Citric Acid.
From courses.lumenlearning.com
Citric Acid Cycle Biology for Majors I Indicator For Citric Acid Citric acid and sodium hydroxide are used for all three presented titrations. Because the indicator’s acid and base forms have different. Some citrus fruits taste more sour, and therefore are more acidic, than others. During the first stages of the titration, the naoh will be completely neutralised, and an. To determine the amount of citric acid present in fruit juices.. Indicator For Citric Acid.
From www.wikidoc.org
Citric acid cycle wikidoc Indicator For Citric Acid During the first stages of the titration, the naoh will be completely neutralised, and an. Citric acid and sodium hydroxide are used for all three presented titrations. Refer to part 1 for the background information and the titration method. Because the indicator’s acid and base forms have different. It is the purpose of this experiment to determine the. Some citrus. Indicator For Citric Acid.
From www.youtube.com
Citric acid cycle regulation YouTube Indicator For Citric Acid In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. Citric acid and sodium hydroxide are used for all three presented titrations. Some citrus fruits taste more sour, and therefore are more acidic, than others. 3 naoh (aq) + h 3 c 6 h 5 o 7. Because. Indicator For Citric Acid.
From healthjade.net
Citric Acid, Citric Acid In Food Uses. Is Citric Acid Bad For You? Indicator For Citric Acid To determine the amount of citric acid present in fruit juices. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. 3 naoh (aq) + h 3 c 6 h 5 o 7. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the. Indicator For Citric Acid.
From www.animalia-life.club
Citric Acid Cycle Indicator For Citric Acid A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. Refer to part 1 for the background information and the titration method. Citric acid and sodium hydroxide are used for all three presented titrations. It is the purpose of this experiment to determine the. To determine the amount of citric acid present in fruit. Indicator For Citric Acid.
From safety365.sevron.co.uk
Citric acid monohydrate MSDS Download Indicator For Citric Acid Citric acid and sodium hydroxide are used for all three presented titrations. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. It is the purpose of this experiment to determine the. Some citrus fruits taste more sour, and therefore are more acidic, than others. During the first. Indicator For Citric Acid.
From chartiereashe1969.blogspot.com
Easy Way to Memorize Mcat Citric Acid Cycle Chartier Eashe1969 Indicator For Citric Acid During the first stages of the titration, the naoh will be completely neutralised, and an. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an. Refer to part 1 for the background information and the titration method. Some citrus fruits taste more sour, and therefore are more acidic,. Indicator For Citric Acid.
From www.indigoinstruments.com
Ascorbic Acid vs Citric Acid Levels with Vitamin C Test Strips Indicator For Citric Acid Citric acid and sodium hydroxide are used for all three presented titrations. Some citrus fruits taste more sour, and therefore are more acidic, than others. During the first stages of the titration, the naoh will be completely neutralised, and an. It is the purpose of this experiment to determine the. To determine the amount of citric acid present in fruit. Indicator For Citric Acid.
From en.wikipedia.org
Citric acid cycle Wikipedia Indicator For Citric Acid Refer to part 1 for the background information and the titration method. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. It is the purpose of this experiment to determine the. 3 naoh (aq) + h 3 c 6 h 5 o 7. Some citrus fruits taste more sour, and therefore are more. Indicator For Citric Acid.
From basicmedicalkey.com
The Citric Acid Cycle The Catabolism of AcetylCoA Basicmedical Key Indicator For Citric Acid Because the indicator’s acid and base forms have different. A suitable indicator has a colour change (endpoint) coinciding with the equivalence point of the titration. During the first stages of the titration, the naoh will be completely neutralised, and an. 3 naoh (aq) + h 3 c 6 h 5 o 7. Citric acid and sodium hydroxide are used for. Indicator For Citric Acid.