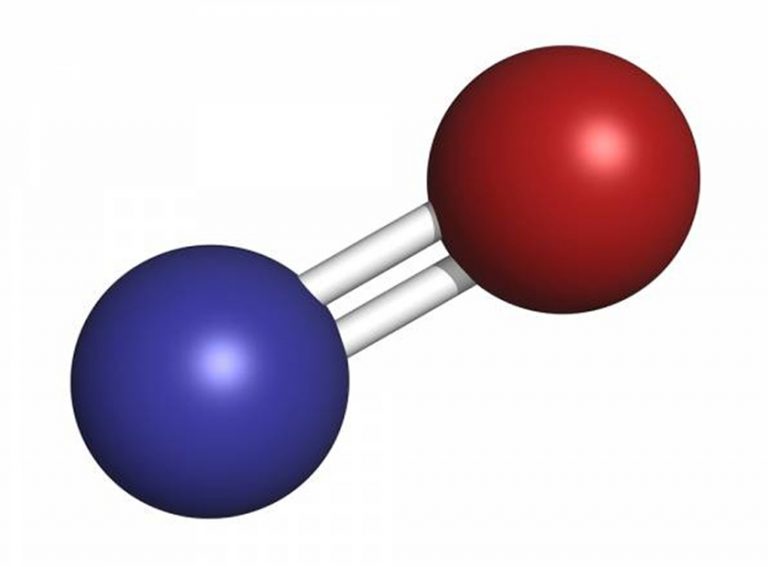
Nitric Oxide Chemical Properties . 2 no + o₂ → 2 no₂; It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). Nitric oxide loves to join forces with oxygen. This brief overview is organized into two general topics, the chemical and the. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. Chemical properties of nitric oxide combines with oxygen. No also reacts with ozone (o₃) in the air. Nitric oxide is a free radical: It has a role as a neurotransmitter, a signalling. Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. This reaction is important in the atmosphere and can affect air quality. When it does, it forms nitrogen dioxide (no2), a brown gas.
from theleader.info
Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). It has a role as a neurotransmitter, a signalling. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. 2 no + o₂ → 2 no₂; No also reacts with ozone (o₃) in the air. This reaction is important in the atmosphere and can affect air quality. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). When it does, it forms nitrogen dioxide (no2), a brown gas. Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. Nitric oxide loves to join forces with oxygen.
What is Nitric Oxide and why does the body need it? The Leader Newspaper
Nitric Oxide Chemical Properties It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). Nitric oxide is a free radical: Chemical properties of nitric oxide combines with oxygen. No also reacts with ozone (o₃) in the air. Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. Nitric oxide loves to join forces with oxygen. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. When it does, it forms nitrogen dioxide (no2), a brown gas. 2 no + o₂ → 2 no₂; It has a role as a neurotransmitter, a signalling. This reaction is important in the atmosphere and can affect air quality. This brief overview is organized into two general topics, the chemical and the. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no).
From www.youtube.com
Chemical properties of Nitric oxide YouTube Nitric Oxide Chemical Properties Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). This reaction is important in the atmosphere and can affect air quality. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). Nitric oxide is a free radical: It has a role. Nitric Oxide Chemical Properties.
From www.vectorstock.com
Nitric oxide molecule skeletal formula Royalty Free Vector Nitric Oxide Chemical Properties When it does, it forms nitrogen dioxide (no2), a brown gas. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). This reaction is important in the atmosphere and can affect air quality. This brief overview is organized into two general topics, the chemical and the. Nitric oxide loves to join forces with. Nitric Oxide Chemical Properties.
From www.youtube.com
Nitric Oxide Preparation, Properties and Uses YouTube Nitric Oxide Chemical Properties It has a role as a neurotransmitter, a signalling. Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. Nitric oxide is a free radical: No also reacts with ozone (o₃) in the air. Nitric oxide loves to join forces with oxygen. 2 no + o₂ →. Nitric Oxide Chemical Properties.
From www.shutterstock.com
Molecular Formula Chemical Structure Nitric Oxide Stock Vector (Royalty Nitric Oxide Chemical Properties Nitric oxide is a free radical: When it does, it forms nitrogen dioxide (no2), a brown gas. It has a role as a neurotransmitter, a signalling. Chemical properties of nitric oxide combines with oxygen. Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. This reaction is. Nitric Oxide Chemical Properties.
From www.schoenfischlab.com
Nitric oxide SCHOENFISCH LAB Nitric Oxide Chemical Properties This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. It has a role as a neurotransmitter, a signalling. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). Chemical properties of nitric oxide combines with. Nitric Oxide Chemical Properties.
From www.slideserve.com
PPT Nitric Oxide PowerPoint Presentation ID183938 Nitric Oxide Chemical Properties When it does, it forms nitrogen dioxide (no2), a brown gas. This brief overview is organized into two general topics, the chemical and the. Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. This structure is also available as a 2d mol file or as a. Nitric Oxide Chemical Properties.
From pediaa.com
Difference Between Nitric Oxide and Nitrous Oxide Definition Nitric Oxide Chemical Properties 2 no + o₂ → 2 no₂; This brief overview is organized into two general topics, the chemical and the. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. It has a role as a neurotransmitter, a signalling. It has an unpaired electron, which is sometimes. Nitric Oxide Chemical Properties.
From www.dreamstime.com
Nitric Oxide, NO, Molecule Model, Chemical Formula. Nitrogen Oxide Nitric Oxide Chemical Properties This brief overview is organized into two general topics, the chemical and the. Nitric oxide loves to join forces with oxygen. Chemical properties of nitric oxide combines with oxygen. This reaction is important in the atmosphere and can affect air quality. No also reacts with ozone (o₃) in the air. It has an unpaired electron, which is sometimes denoted by. Nitric Oxide Chemical Properties.
From www.researchgate.net
Chemical equations relevant to the nitric oxide system. Download Nitric Oxide Chemical Properties Nitric oxide loves to join forces with oxygen. Chemical properties of nitric oxide combines with oxygen. Nitric oxide is a free radical: No also reacts with ozone (o₃) in the air. 2 no + o₂ → 2 no₂; Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen. Nitric Oxide Chemical Properties.
From www.shutterstock.com
Chemical Formulas Molecule Model Nitrogen Oxide Stock Vector (Royalty Nitric Oxide Chemical Properties This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. This brief overview is organized into two general topics, the chemical and the. Nitric oxide loves to join forces with oxygen. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric Oxide Chemical Properties.
From integrativemedicinepa.com
The Nitric Oxide Miracle Molecule — Integrative Medicine Solutions Nitric Oxide Chemical Properties When it does, it forms nitrogen dioxide (no2), a brown gas. Nitric oxide is a free radical: This reaction is important in the atmosphere and can affect air quality. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. Chemical properties of nitric oxide combines with oxygen.. Nitric Oxide Chemical Properties.
From fphoto.photoshelter.com
nitric oxide gas chemistry vapor Fundamental Photographs The Art of Nitric Oxide Chemical Properties Chemical properties of nitric oxide combines with oxygen. This reaction is important in the atmosphere and can affect air quality. Nitric oxide loves to join forces with oxygen. It has a role as a neurotransmitter, a signalling. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). When it does, it forms nitrogen. Nitric Oxide Chemical Properties.
From www.youtube.com
Molecular Orbital Diagram for Nitric Oxide Heteronuclear molecule YouTube Nitric Oxide Chemical Properties Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. 2 no + o₂ → 2 no₂; This reaction is important in the atmosphere. Nitric Oxide Chemical Properties.
From www.alamy.com
Nitric oxide, NO, molecule model and chemical formula. Nitrogen oxide Nitric Oxide Chemical Properties It has a role as a neurotransmitter, a signalling. When it does, it forms nitrogen dioxide (no2), a brown gas. Chemical properties of nitric oxide combines with oxygen. Nitric oxide is a free radical: This brief overview is organized into two general topics, the chemical and the. This structure is also available as a 2d mol file or as a. Nitric Oxide Chemical Properties.
From www.nitricoxidesociety.org
Resources — Nitric Oxide Society Nitric Oxide Chemical Properties This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. Nitric oxide is a free radical: Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. Nitric oxide loves to join forces with oxygen.. Nitric Oxide Chemical Properties.
From www.alamy.com
Chemical formulas of nitrogen oxide nitric oxide NO, nitrogen dioxide Nitric Oxide Chemical Properties This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. This brief overview is organized into two general topics, the chemical and the. It. Nitric Oxide Chemical Properties.
From www.dreamstime.com
Nitric Oxide, NO, Molecule Model, Chemical Formula. Nitrogen Oxide Nitric Oxide Chemical Properties When it does, it forms nitrogen dioxide (no2), a brown gas. This brief overview is organized into two general topics, the chemical and the. It has a role as a neurotransmitter, a signalling. No also reacts with ozone (o₃) in the air. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o. Nitric Oxide Chemical Properties.
From www.dreamstime.com
3D Image of Nitric Oxide Skeletal Formula Stock Illustration Nitric Oxide Chemical Properties 2 no + o₂ → 2 no₂; This brief overview is organized into two general topics, the chemical and the. Nitric oxide is a free radical: It has a role as a neurotransmitter, a signalling. Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. This structure. Nitric Oxide Chemical Properties.
From ar.inspiredpencil.com
Nitric Oxide Lewis Structure Nitric Oxide Chemical Properties Nitric oxide is a free radical: No also reacts with ozone (o₃) in the air. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed. Nitric oxide loves to join forces with oxygen. Chemical properties of nitric oxide combines with oxygen. Nitric oxide is a relatively unstable,. Nitric Oxide Chemical Properties.
From melscience.com
Methods of synthesis of nitrous oxide MEL Chemistry Nitric Oxide Chemical Properties 2 no + o₂ → 2 no₂; Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). Nitric oxide is a free radical: It has a role as a neurotransmitter,. Nitric Oxide Chemical Properties.
From c10.beauty
Nitric Oxide Molecule Structure Nitric Oxide Chemical Properties Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). No also reacts with ozone (o₃) in the air. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). 2 no + o₂ → 2 no₂; This brief overview is organized into. Nitric Oxide Chemical Properties.
From pediaa.com
Difference Between Nitric Oxide and Nitrous Oxide Definition Nitric Oxide Chemical Properties Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. It has a role as a neurotransmitter, a signalling. It has an unpaired electron, which is sometimes denoted by a. Nitric Oxide Chemical Properties.
From ar.inspiredpencil.com
Nitric Oxide Lewis Structure Nitric Oxide Chemical Properties No also reacts with ozone (o₃) in the air. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). 2 no + o₂ → 2 no₂; This brief overview is organized into two general topics, the chemical and the. Chemical properties of nitric oxide combines with oxygen. Nitric oxide. Nitric Oxide Chemical Properties.
From www.researchgate.net
Chemical equations relevant to the nitric oxide system. Download Nitric Oxide Chemical Properties Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). Nitric oxide is a free radical: It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). When it does, it forms nitrogen dioxide (no2), a brown gas. No also reacts with ozone. Nitric Oxide Chemical Properties.
From classnotes.org.in
Oxides of Nitrogen Chemistry, Class 12, The pBlock Elements Nitric Oxide Chemical Properties It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). This brief overview is organized into two general topics, the chemical and the. Chemical properties of nitric oxide combines with oxygen. This reaction is important in the atmosphere and can affect air quality. Nitric oxide is a free radical:. Nitric Oxide Chemical Properties.
From www.youtube.com
LECTURE7 OXIDES OF NITROGEN NITROUS OXIDE, NITRIC OXIDE, CHEMICAL Nitric Oxide Chemical Properties Nitric oxide is a free radical: It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. No also reacts with ozone (o₃) in the air. Nitric. Nitric Oxide Chemical Properties.
From www.researchgate.net
1 The various routes of nitric oxide formation in plants. Reductive Nitric Oxide Chemical Properties Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom. No also reacts with ozone (o₃) in the air. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). It has an unpaired electron, which is sometimes denoted by a. Nitric Oxide Chemical Properties.
From www.vectorstock.com
Nitric oxide molecule skeletal formula Royalty Free Vector Nitric Oxide Chemical Properties When it does, it forms nitrogen dioxide (no2), a brown gas. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). It has a role as a neurotransmitter, a signalling. Chemical properties of nitric oxide combines with oxygen. This reaction is important in the atmosphere and can affect air quality. This brief overview. Nitric Oxide Chemical Properties.
From mungfali.com
Nitric Oxide Lewis Structure Nitric Oxide Chemical Properties This brief overview is organized into two general topics, the chemical and the. When it does, it forms nitrogen dioxide (no2), a brown gas. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). 2 no + o₂ → 2 no₂; Nitric oxide is a relatively unstable, diatomic molecule. Nitric Oxide Chemical Properties.
From www.slideserve.com
PPT Nitric Oxide PowerPoint Presentation, free download ID4751599 Nitric Oxide Chemical Properties Nitric oxide loves to join forces with oxygen. No also reacts with ozone (o₃) in the air. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). 2 no + o₂ → 2 no₂; Nitric oxide is a nitrogen oxide which is a free radical, each molecule of which. Nitric Oxide Chemical Properties.
From www.alamy.com
Nitrous oxide molecule hires stock photography and images Alamy Nitric Oxide Chemical Properties Nitric oxide is a free radical: Nitric oxide loves to join forces with oxygen. It has a role as a neurotransmitter, a signalling. This brief overview is organized into two general topics, the chemical and the. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). This reaction is important in the atmosphere. Nitric Oxide Chemical Properties.
From theleader.info
What is Nitric Oxide and why does the body need it? The Leader Newspaper Nitric Oxide Chemical Properties This reaction is important in the atmosphere and can affect air quality. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). No also reacts with ozone (o₃) in the air. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). Nitric. Nitric Oxide Chemical Properties.
From www.slideserve.com
PPT Nitric Oxide PowerPoint Presentation, free download ID183938 Nitric Oxide Chemical Properties 2 no + o₂ → 2 no₂; Nitric oxide is a free radical: Chemical properties of nitric oxide combines with oxygen. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). Nitric oxide loves to join forces with oxygen. It has a role as a neurotransmitter, a signalling. When. Nitric Oxide Chemical Properties.
From present5.com
Biological Effects of Nitric Oxide and its Role Nitric Oxide Chemical Properties 2 no + o₂ → 2 no₂; When it does, it forms nitrogen dioxide (no2), a brown gas. This reaction is important in the atmosphere and can affect air quality. Nitric oxide loves to join forces with oxygen. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). This structure is also available. Nitric Oxide Chemical Properties.
From pubs.acs.org
Connecting the Chemical and Biological Properties of Nitric Oxide Nitric Oxide Chemical Properties Chemical properties of nitric oxide combines with oxygen. Nitric oxide is a relatively unstable, diatomic molecule that possesses a free radical (i.e., an unpaired electron). This reaction is important in the atmosphere and can affect air quality. It has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (• n=o or • no). When it. Nitric Oxide Chemical Properties.