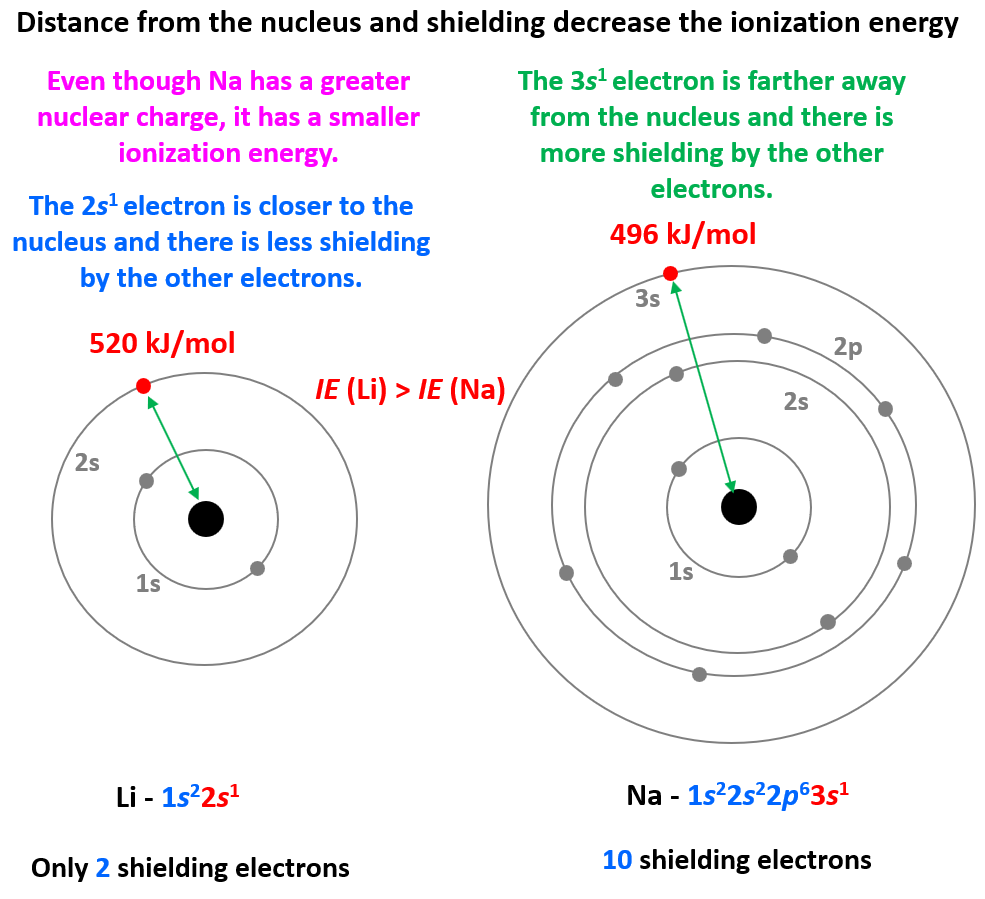
What Does Electron Shielding Mean . When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells.
from ar.inspiredpencil.com
The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent.
Electron Shielding
What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From ar.inspiredpencil.com
Electron Shielding What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.slideserve.com
PPT Chapter 8 Periodic Properties & Electron Configurations Bushra What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From wisc.pb.unizin.org
Core and Valence Electrons, Shielding, Zeff (M7Q8) UWMadison What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they. What Does Electron Shielding Mean.
From www.youtube.com
Electron Shielding Groups YouTube What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they. What Does Electron Shielding Mean.
From byjus.com
What is shielding and deshielding in NMR? Give an example? What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From www.youtube.com
Electron shielding YouTube What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they. What Does Electron Shielding Mean.
From ar.inspiredpencil.com
Electron Shielding What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID2836425 What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.slideserve.com
PPT Chapter 6 Periodic Table PowerPoint Presentation, free download What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From slideplayer.com
Coulomb’s Law PERIODIC TRENDS Nuclear Charge Electron Shielding ppt What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they. What Does Electron Shielding Mean.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.slideserve.com
PPT The Development of the Periodic Table PowerPoint Presentation What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they. What Does Electron Shielding Mean.
From www.youtube.com
Chapter 3 Shielding Effect and Atomic Size YouTube What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From www.youtube.com
nuclear charge and electron shielding YouTube What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.youtube.com
Shielding effect of Electroninner Electrons on outer Electronin the What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they. What Does Electron Shielding Mean.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID1588021 What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they. What Does Electron Shielding Mean.
From www.nuclear-power.com
Shielding of Alpha Radiation Types & Uses What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they. What Does Electron Shielding Mean.
From www.nuclear-power.com
Shielding of Neutron Radiation Types & Uses What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear. What Does Electron Shielding Mean.
From www.youtube.com
Zeff and Electron Shielding YouTube What Does Electron Shielding Mean Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they. What Does Electron Shielding Mean.
From www.youtube.com
Orbital shielding and YouTube What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Does Electron Shielding Mean When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e What Does Electron Shielding Mean The shielding effect, also known as the screening effect, is the decrease in the nuclear attraction on the valence shell caused by the presence of electrons in the inner shells. When there are more inner electrons, they shield the outermost electron from the nucleus and neglect the nuclear attraction to some extent. Electrons in an \(s\) orbital can shield \(p\). What Does Electron Shielding Mean.