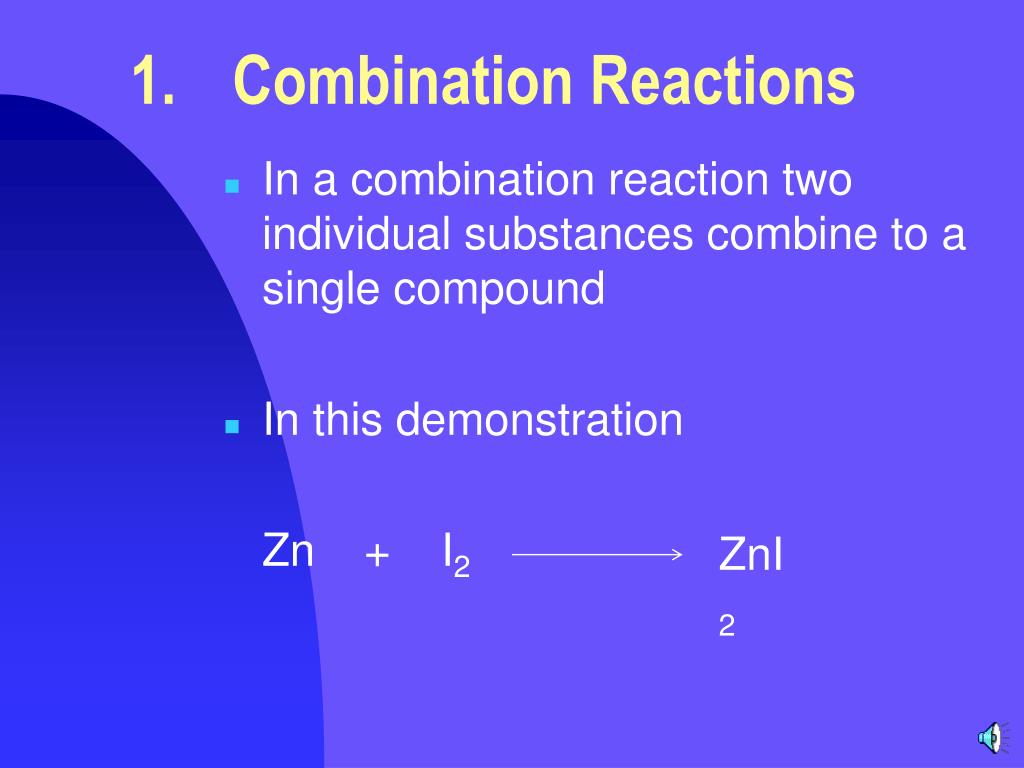
Zinc And Iodine Reaction Balanced Equation . Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Diiodine + zinc = zinc iodide. Write and balance chemical equations in molecular, total. Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Derive chemical equations from narrative descriptions of chemical reactions. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zn + i 2 → zni 2 Zinc + iodine = zinc iodide. Chemical equation (h2o = h + o) 🛠️
from www.slideserve.com
Chemical equation (h2o = h + o) 🛠️ Diiodine + zinc = zinc iodide. Zinc + iodine = zinc iodide. Zn + i 2 → zni 2 Write and balance chemical equations in molecular, total. Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Derive chemical equations from narrative descriptions of chemical reactions. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Use the calculator below to balance chemical equations and determine the type of reaction (instructions).
PPT Reaction of Zinc and Iodine PowerPoint Presentation, free
Zinc And Iodine Reaction Balanced Equation Zinc + iodine = zinc iodide. Write and balance chemical equations in molecular, total. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Diiodine + zinc = zinc iodide. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Derive chemical equations from narrative descriptions of chemical reactions. Zinc + iodine = zinc iodide. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Chemical equation (h2o = h + o) 🛠️ Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i 2 → zni 2
From www.toppr.com
In the reaction between zinc and iodine , zinc iodide is formed . What Zinc And Iodine Reaction Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Write and balance chemical equations in molecular, total. Zn + i 2 → zni 2 Zinc + iodine = zinc iodide. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Zinc. Zinc And Iodine Reaction Balanced Equation.
From www.toppr.com
Balanced equation for the reaction between zinc and dilute sulphuric Zinc And Iodine Reaction Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zinc + iodine = zinc iodide. Diiodine + zinc = zinc iodide. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zinc powder is added to a solution of iodine in ethanol. Write and balance chemical equations in. Zinc And Iodine Reaction Balanced Equation.
From www.youtube.com
How to Balance AgNO3 + ZnI2 = AgI + Zn(NO3)2 Silver Nitrate + Zinc Zinc And Iodine Reaction Balanced Equation Write and balance chemical equations in molecular, total. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i 2 → zni 2 I2 + zn = zni2 is. Zinc And Iodine Reaction Balanced Equation.
From www.chegg.com
Solved Choose balanced molecular, ionic, and net ionic Zinc And Iodine Reaction Balanced Equation Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Write and balance chemical equations in molecular, total. Zinc + iodine = zinc iodide. Diiodine + zinc = zinc iodide. Zinc powder is added to a solution of iodine in ethanol. Use the calculator below to balance. Zinc And Iodine Reaction Balanced Equation.
From www.youtube.com
How to Write the Formula for Zinc iodide YouTube Zinc And Iodine Reaction Balanced Equation Diiodine + zinc = zinc iodide. Zn + i 2 → zni 2 Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zinc powder is added to a solution of iodine in ethanol. Chemical equation (h2o = h + o) 🛠️ Write and balance chemical equations in molecular, total. An exothermic redox reaction occurs,. Zinc And Iodine Reaction Balanced Equation.
From www.slideserve.com
PPT Reaction of Zinc and Iodine PowerPoint Presentation, free Zinc And Iodine Reaction Balanced Equation Zinc + iodine = zinc iodide. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Zn + i 2 → zni 2 Chemical equation (h2o = h + o) 🛠️ Write and balance chemical equations in molecular, total. Use the calculator below to balance chemical equations. Zinc And Iodine Reaction Balanced Equation.
From exotwicqs.blob.core.windows.net
Iodine And Zinc Equation at Nancy Moore blog Zinc And Iodine Reaction Balanced Equation I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to.. Zinc And Iodine Reaction Balanced Equation.
From lessonlistinvincibly.z13.web.core.windows.net
Zinc And Iodine Reaction Zinc And Iodine Reaction Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Derive chemical equations from narrative descriptions of chemical reactions. Write and balance chemical equations in molecular, total. Diiodine + zinc = zinc iodide. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine. Zinc And Iodine Reaction Balanced Equation.
From www.toppr.com
Write the balanced chemical reaction for the following and identify the Zinc And Iodine Reaction Balanced Equation I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zn + i 2 → zni 2 Diiodine + zinc = zinc iodide. Write and balance chemical equations in molecular, total. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zn +. Zinc And Iodine Reaction Balanced Equation.
From www.coursehero.com
[Solved] Data analysis reaction of zinc and iodine (limiting reactant Zinc And Iodine Reaction Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Write and balance chemical equations in molecular, total. Zn + i 2 → zni 2 Chemical equation (h2o = h + o). Zinc And Iodine Reaction Balanced Equation.
From www.coursehero.com
[Solved] Data analysis reaction of zinc and iodine (limiting reactant Zinc And Iodine Reaction Balanced Equation Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zn + i 2 → zni 2 Zinc + iodine = zinc iodide. Chemical equation. Zinc And Iodine Reaction Balanced Equation.
From www.numerade.com
SOLVED Zinc reacts with iodine in a synthesis reaction. Using a Zinc And Iodine Reaction Balanced Equation Write and balance chemical equations in molecular, total. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Diiodine + zinc = zinc iodide. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Derive chemical equations from narrative descriptions of chemical reactions. I2 + zn = zni2 is. Zinc And Iodine Reaction Balanced Equation.
From www.coursehero.com
[Solved] Data analysis reaction of zinc and iodine (limiting reactant Zinc And Iodine Reaction Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zinc powder is added to a solution of iodine in ethanol. Zinc + iodine = zinc iodide. Chemical equation (h2o = h + o) 🛠️ I2 + zn =. Zinc And Iodine Reaction Balanced Equation.
From www.numerade.com
SOLVED Write balanced complete chemical equation the complete ionic Zinc And Iodine Reaction Balanced Equation I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i 2 → zni 2 Write and balance chemical equations in molecular, total. Zn + i = zni2 is a. Zinc And Iodine Reaction Balanced Equation.
From www.slideserve.com
PPT Reaction of Zinc and Iodine PowerPoint Presentation, free Zinc And Iodine Reaction Balanced Equation Write and balance chemical equations in molecular, total. Chemical equation (h2o = h + o) 🛠️ Diiodine + zinc = zinc iodide. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Derive chemical equations from narrative descriptions of chemical reactions. I2 + zn = zni2 is a synthesis reaction where one mole of. Zinc And Iodine Reaction Balanced Equation.
From www.numerade.com
SOLVED Zinc reacts with iodine in a synthesis reaction. Using a Zinc And Iodine Reaction Balanced Equation Zn + i 2 → zni 2 Write and balance chemical equations in molecular, total. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Derive chemical equations from narrative descriptions of chemical reactions. Use the calculator below to balance chemical equations and determine the type of reaction. Zinc And Iodine Reaction Balanced Equation.
From www.numerade.com
SOLVED The reaction of iodine with zinc observations. Zinc And Iodine Reaction Balanced Equation Zinc powder is added to a solution of iodine in ethanol. Chemical equation (h2o = h + o) 🛠️ Zinc + iodine = zinc iodide. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine. Zinc And Iodine Reaction Balanced Equation.
From www.slideserve.com
PPT Chemical Reactions An Introduction PowerPoint Presentation, free Zinc And Iodine Reaction Balanced Equation Zinc powder is added to a solution of iodine in ethanol. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zinc + iodine = zinc iodide. Derive chemical equations from narrative descriptions of chemical reactions. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained. Zinc And Iodine Reaction Balanced Equation.
From www.coursehero.com
[Solved] Data analysis reaction of zinc and iodine (limiting reactant Zinc And Iodine Reaction Balanced Equation Zinc + iodine = zinc iodide. Chemical equation (h2o = h + o) 🛠️ Zinc powder is added to a solution of iodine in ethanol. Diiodine + zinc = zinc iodide. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. An exothermic redox reaction occurs, forming zinc. Zinc And Iodine Reaction Balanced Equation.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc And Iodine Reaction Balanced Equation Zn + i 2 → zni 2 Derive chemical equations from narrative descriptions of chemical reactions. Chemical equation (h2o = h + o) 🛠️ Zinc + iodine = zinc iodide. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zinc powder is added to a solution of iodine in ethanol. Diiodine + zinc. Zinc And Iodine Reaction Balanced Equation.
From www.chegg.com
Solved Zinc is a metal and iodine is a nonmetal. Write a Zinc And Iodine Reaction Balanced Equation Chemical equation (h2o = h + o) 🛠️ Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zinc +. Zinc And Iodine Reaction Balanced Equation.
From www.numerade.com
SOLVED What is the expected empirical formula for the compound that Zinc And Iodine Reaction Balanced Equation Write and balance chemical equations in molecular, total. Zinc powder is added to a solution of iodine in ethanol. Derive chemical equations from narrative descriptions of chemical reactions. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Diiodine + zinc = zinc iodide. I2 + zn = zni2 is a synthesis reaction where. Zinc And Iodine Reaction Balanced Equation.
From edu-rsc-org-ssl.oca.korea.ac.kr
Exothermic redox reaction of zinc with iodine Experiment RSC Education Zinc And Iodine Reaction Balanced Equation Zinc + iodine = zinc iodide. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Derive chemical equations from narrative descriptions of chemical reactions. Zn + i 2 → zni 2 Write and balance chemical equations in molecular, total. Use the calculator below to balance chemical. Zinc And Iodine Reaction Balanced Equation.
From www.numerade.com
SOLVED A Synthesis and formula of zinc iodide 1 Write the expected Zinc And Iodine Reaction Balanced Equation Write and balance chemical equations in molecular, total. Zinc powder is added to a solution of iodine in ethanol. Zinc + iodine = zinc iodide. Derive chemical equations from narrative descriptions of chemical reactions. Chemical equation (h2o = h + o) 🛠️ Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zn + i. Zinc And Iodine Reaction Balanced Equation.
From www.numerade.com
SOLVED (a) Write an equation for the formation of zinc iodide from Zinc And Iodine Reaction Balanced Equation Write and balance chemical equations in molecular, total. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. I2 + zn = zni2 is a synthesis reaction where. Zinc And Iodine Reaction Balanced Equation.
From www.sciencephoto.com
Zinc reacts with iodine Stock Image C044/0436 Science Photo Library Zinc And Iodine Reaction Balanced Equation Zinc + iodine = zinc iodide. Zinc powder is added to a solution of iodine in ethanol. Diiodine + zinc = zinc iodide. Derive chemical equations from narrative descriptions of chemical reactions. Write and balance chemical equations in molecular, total. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. I2 + zn =. Zinc And Iodine Reaction Balanced Equation.
From www.numerade.com
SOLVED BALANCING CHEMICAL EQUATIONS HYDROGEN GAS OXYGEN GAS WATER Zinc And Iodine Reaction Balanced Equation Zinc powder is added to a solution of iodine in ethanol. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Use the calculator below. Zinc And Iodine Reaction Balanced Equation.
From www.toppr.com
In the reaction between zinc and iodine, zinc iodide is formed. Which Zinc And Iodine Reaction Balanced Equation Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Derive chemical equations from narrative descriptions of chemical reactions. Write and balance chemical equations in molecular, total. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and. Zinc And Iodine Reaction Balanced Equation.
From www.researchgate.net
(PDF) Synthesis and of Zinc Iodide Model Reactions for Zinc And Iodine Reaction Balanced Equation Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Chemical equation (h2o = h + o) 🛠️ Write and balance chemical equations in molecular, total. Derive chemical equations from narrative descriptions of chemical reactions. Diiodine + zinc = zinc iodide. Use the calculator below to balance. Zinc And Iodine Reaction Balanced Equation.
From mmeijerchemreactions.blogspot.com
Chemical Reactions Zinc And Iodine Lab Zinc And Iodine Reaction Balanced Equation Derive chemical equations from narrative descriptions of chemical reactions. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Zinc powder is added to a solution of iodine in ethanol. Chemical equation (h2o = h + o) 🛠️ Write and balance chemical equations in molecular, total. I2 + zn = zni2 is a synthesis reaction. Zinc And Iodine Reaction Balanced Equation.
From www.numerade.com
SOLVED Synthesis and formula of zinc iodide Write the expected Zinc And Iodine Reaction Balanced Equation I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Write and balance chemical equations in molecular, total. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Derive chemical equations from narrative descriptions of. Zinc And Iodine Reaction Balanced Equation.
From www.numerade.com
SOLVED A voltaic cell prepared using zinc and iodine has the following Zinc And Iodine Reaction Balanced Equation An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Derive chemical equations from narrative descriptions of chemical reactions. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Use the calculator below to balance chemical equations and determine the type. Zinc And Iodine Reaction Balanced Equation.
From www.slideserve.com
PPT Reaction of Zinc and Iodine PowerPoint Presentation, free Zinc And Iodine Reaction Balanced Equation An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zinc powder is added to a solution of iodine in ethanol. Zn + i 2 → zni 2 Write and balance chemical equations in molecular, total. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one. Zinc And Iodine Reaction Balanced Equation.
From www.slideserve.com
PPT Reaction of Zinc and Iodine PowerPoint Presentation, free Zinc And Iodine Reaction Balanced Equation I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Diiodine + zinc = zinc iodide. Zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to. Write and balance chemical equations in molecular, total. Derive. Zinc And Iodine Reaction Balanced Equation.
From www.slideserve.com
PPT Reaction of Zinc and Iodine PowerPoint Presentation, free Zinc And Iodine Reaction Balanced Equation Chemical equation (h2o = h + o) 🛠️ Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Derive chemical equations from narrative descriptions of chemical reactions. Zinc powder is added to a solution of iodine in ethanol. Zinc + iodine = zinc iodide. Write and balance chemical equations in molecular, total. Zn + i. Zinc And Iodine Reaction Balanced Equation.