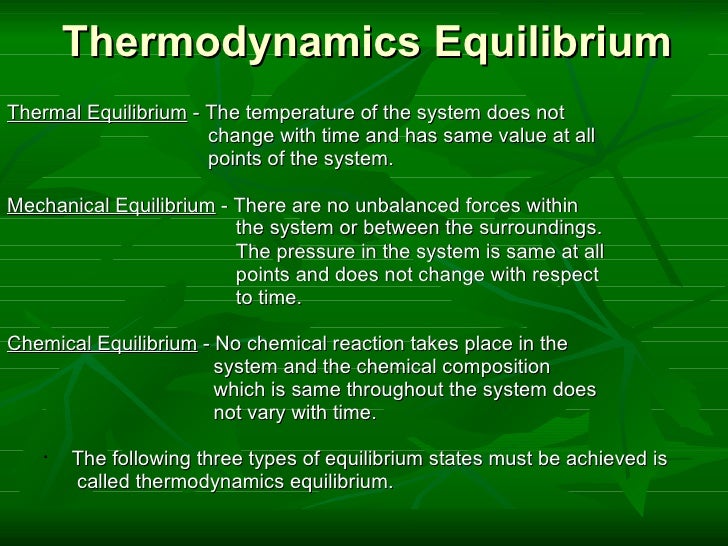
Mechanical Equilibrium And Thermal . 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. an important concept related to temperature is thermal equilibrium. if two systems can exchange energy, then t ∗ 1 = t ∗ 2. a thermometer measures its own temperature. Two objects are in thermal equilibrium if. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. An important concept related to temperature is thermal equilibrium. Two objects are in thermal equilibrium if they are in close. If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. It is through the concepts of thermal equilibrium and the zeroth law of.
from www.slideshare.net
Two objects are in thermal equilibrium if they are in close. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. if two systems can exchange energy, then t ∗ 1 = t ∗ 2. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. Two objects are in thermal equilibrium if. an important concept related to temperature is thermal equilibrium. An important concept related to temperature is thermal equilibrium. a thermometer measures its own temperature.
Thermodynamics
Mechanical Equilibrium And Thermal An important concept related to temperature is thermal equilibrium. Two objects are in thermal equilibrium if. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. an important concept related to temperature is thermal equilibrium. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. if two systems can exchange energy, then t ∗ 1 = t ∗ 2. If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. It is through the concepts of thermal equilibrium and the zeroth law of. An important concept related to temperature is thermal equilibrium. a thermometer measures its own temperature. Two objects are in thermal equilibrium if they are in close.
From lawofthermodynamicsinfo.com
What Is Thermodynamic Equilibrium? (With Best Examples) Mechanical Equilibrium And Thermal a thermometer measures its own temperature. It is through the concepts of thermal equilibrium and the zeroth law of. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. Two objects are in thermal equilibrium if they are in close. If two systems can exchange particle number,. Mechanical Equilibrium And Thermal.
From exyaptclg.blob.core.windows.net
What Does Mechanical Equilibrium Mean at Hazel Dean blog Mechanical Equilibrium And Thermal an important concept related to temperature is thermal equilibrium. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. If two systems can exchange particle number, then μ. Mechanical Equilibrium And Thermal.
From www.youtube.com
Thermodynamics Equilibrium Zeroth Law of thermodynamics Steady Mechanical Equilibrium And Thermal It is through the concepts of thermal equilibrium and the zeroth law of. if two systems can exchange energy, then t ∗ 1 = t ∗ 2. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. an important concept related to temperature is thermal equilibrium.. Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT Part 2 Statistical Mechanics PowerPoint Presentation, free Mechanical Equilibrium And Thermal an important concept related to temperature is thermal equilibrium. If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. An important concept related to temperature is thermal equilibrium. Two objects are in thermal equilibrium if. thermal equilibrium is established when two bodies are in thermal. Mechanical Equilibrium And Thermal.
From slidetodoc.com
Physics 101 Lecture 13 Thermal Physics Thermodynamics Third Mechanical Equilibrium And Thermal An important concept related to temperature is thermal equilibrium. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. an important concept related to temperature is thermal equilibrium. It is through the concepts of thermal equilibrium and the zeroth law of. if two systems can exchange. Mechanical Equilibrium And Thermal.
From www.numerade.com
Energy can be transferred from a system to its surroundings as work if Mechanical Equilibrium And Thermal An important concept related to temperature is thermal equilibrium. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. an important concept related to temperature is thermal equilibrium.. Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT Thermal Physics PowerPoint Presentation, free download ID9512945 Mechanical Equilibrium And Thermal An important concept related to temperature is thermal equilibrium. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. Two objects are in thermal equilibrium if. It is through the concepts of thermal equilibrium and the zeroth law of. 1 temperature and thermal equilibrium when we speak. Mechanical Equilibrium And Thermal.
From www.youtube.com
09 conditions of equilibriumthermal , mechanical and particle Mechanical Equilibrium And Thermal An important concept related to temperature is thermal equilibrium. an important concept related to temperature is thermal equilibrium. Two objects are in thermal equilibrium if they are in close. a thermometer measures its own temperature. Two objects are in thermal equilibrium if. It is through the concepts of thermal equilibrium and the zeroth law of. 1 temperature. Mechanical Equilibrium And Thermal.
From www.youtube.com
1.5 System Equilibrium Mechanical equilibrium Thermal equilibrium Mechanical Equilibrium And Thermal if two systems can exchange energy, then t ∗ 1 = t ∗ 2. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. . Mechanical Equilibrium And Thermal.
From www.youtube.com
Equilibrium Thermal ,Chemical, Mechanical & Thermodynamic Basic Mechanical Equilibrium And Thermal Two objects are in thermal equilibrium if they are in close. a thermometer measures its own temperature. Two objects are in thermal equilibrium if. If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. an important concept related to temperature is thermal equilibrium. if. Mechanical Equilibrium And Thermal.
From www.worksheetsplanet.com
What is Thermal Equilibrium Mechanical Equilibrium And Thermal If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. Two objects are in thermal equilibrium if they are in close. Two objects are in thermal equilibrium if. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the.. Mechanical Equilibrium And Thermal.
From www.youtube.com
10. Mechanical Equilibrium and Pressure Thermal and Statistical Mechanical Equilibrium And Thermal 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. an important concept related to temperature is thermal equilibrium. thermal equilibrium is established. Mechanical Equilibrium And Thermal.
From www.geeksforgeeks.org
What is Thermodynamics Definition, Laws, Formulas, Class 11 Notes Mechanical Equilibrium And Thermal If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. a thermometer measures its own temperature. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. if two systems can exchange energy, then t ∗ 1. Mechanical Equilibrium And Thermal.
From www.youtube.com
Heat Flow & Thermal Equilibrium YouTube Mechanical Equilibrium And Thermal a thermometer measures its own temperature. an important concept related to temperature is thermal equilibrium. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. Two. Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT Heat and heat transfer PowerPoint Presentation, free download Mechanical Equilibrium And Thermal Two objects are in thermal equilibrium if. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. a thermometer measures its own temperature. Two objects are in thermal equilibrium if they are in close. thermal equilibrium is established when two bodies are in thermal contact with. Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT Lecture N. 8 Thermodynamics (concepts, definitions, and basic Mechanical Equilibrium And Thermal Two objects are in thermal equilibrium if they are in close. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. a thermometer measures its own temperature. if two systems can exchange energy, then t ∗ 1 = t ∗ 2. Two objects are in thermal equilibrium if. . Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT Lecture Notes Week 1 PowerPoint Presentation, free download ID Mechanical Equilibrium And Thermal a thermometer measures its own temperature. Two objects are in thermal equilibrium if. an important concept related to temperature is thermal equilibrium. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. It is through the concepts of thermal equilibrium and the zeroth law of. If. Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT A7. Basics of Thermodynamics PowerPoint Presentation, free Mechanical Equilibrium And Thermal Two objects are in thermal equilibrium if they are in close. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. An important concept related to temperature is thermal. Mechanical Equilibrium And Thermal.
From thermalengineeringlearn.blogspot.com
THERMAL ENGINEERING Thermodynamic Equilibrium Mechanical Equilibrium And Thermal a thermometer measures its own temperature. an important concept related to temperature is thermal equilibrium. if two systems can exchange energy, then t ∗ 1 = t ∗ 2. It is through the concepts of thermal equilibrium and the zeroth law of. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning. Mechanical Equilibrium And Thermal.
From www.youtube.com
Thermodynamic equilibrium ( mechanical, chemical and thermal) YouTube Mechanical Equilibrium And Thermal Two objects are in thermal equilibrium if. a thermometer measures its own temperature. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. It is through the concepts. Mechanical Equilibrium And Thermal.
From www.slideshare.net
Thermodynamics Mechanical Equilibrium And Thermal if two systems can exchange energy, then t ∗ 1 = t ∗ 2. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. a thermometer measures. Mechanical Equilibrium And Thermal.
From www.youtube.com
Thermodynamic Equilibriums Thermal Equilibrium Mechanical Mechanical Equilibrium And Thermal if two systems can exchange energy, then t ∗ 1 = t ∗ 2. Two objects are in thermal equilibrium if. If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. Two objects are in thermal equilibrium if they are in close. 1 temperature and. Mechanical Equilibrium And Thermal.
From www.youtube.com
Mechanical equilibrium YouTube Mechanical Equilibrium And Thermal Two objects are in thermal equilibrium if. An important concept related to temperature is thermal equilibrium. a thermometer measures its own temperature. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. It is through the concepts of thermal equilibrium and the zeroth law of. If two systems can exchange. Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT Lecture Notes Week 1 PowerPoint Presentation, free download ID Mechanical Equilibrium And Thermal 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. an important concept related to temperature is thermal equilibrium. if two systems can. Mechanical Equilibrium And Thermal.
From www.scribd.com
Basic Thermodynamics Mechanical and Thermal Equilibrium PDF Gases Mechanical Equilibrium And Thermal Two objects are in thermal equilibrium if. It is through the concepts of thermal equilibrium and the zeroth law of. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. an important concept related to temperature is thermal equilibrium. if two systems can exchange energy, then t ∗ 1. Mechanical Equilibrium And Thermal.
From brainly.in
What are mechanical equilibrium and thermal equilibrium? Brainly.in Mechanical Equilibrium And Thermal 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat. Mechanical Equilibrium And Thermal.
From www.studypool.com
SOLUTION Equilibrium types of equilibrium , thermal equilibrium Mechanical Equilibrium And Thermal an important concept related to temperature is thermal equilibrium. if two systems can exchange energy, then t ∗ 1 = t ∗ 2. a thermometer measures its own temperature. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. thermal equilibrium is established when. Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT Thermal Energy PowerPoint Presentation, free download ID1452353 Mechanical Equilibrium And Thermal Two objects are in thermal equilibrium if they are in close. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. if two systems can exchange energy, then. Mechanical Equilibrium And Thermal.
From www.youtube.com
Heat transfer and thermal equilibrium Thermodynamics AP Chemistry Mechanical Equilibrium And Thermal a thermometer measures its own temperature. An important concept related to temperature is thermal equilibrium. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. It is through the concepts of thermal equilibrium and the zeroth law of. Two objects are in thermal equilibrium if they are. Mechanical Equilibrium And Thermal.
From mavink.com
Thermal Equilibrium Diagram Mechanical Equilibrium And Thermal thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. if two systems can exchange energy, then t ∗ 1 = t ∗ 2. 1 temperature and. Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT MEL140 PowerPoint Presentation, free download ID2475065 Mechanical Equilibrium And Thermal If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. It is through the concepts of thermal equilibrium and the zeroth law of. a thermometer measures its. Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT Lecture N. 8 Thermodynamics (concepts, definitions, and basic Mechanical Equilibrium And Thermal a thermometer measures its own temperature. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 = p ∗ 2 / t ∗ 2. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. thermal equilibrium is established when two bodies are. Mechanical Equilibrium And Thermal.
From www.grc.nasa.gov
First Law of Thermodynamics Mechanical Equilibrium And Thermal a thermometer measures its own temperature. if two systems can exchange energy, then t ∗ 1 = t ∗ 2. If two systems can exchange particle number, then μ ∗ 1 / t ∗ 1 = μ ∗ 2 / t ∗ 2. If two systems can exchange volume, then p ∗ 1 / t ∗ 1 =. Mechanical Equilibrium And Thermal.
From www.slideserve.com
PPT Chem 300 PowerPoint Presentation, free download Mechanical Equilibrium And Thermal an important concept related to temperature is thermal equilibrium. An important concept related to temperature is thermal equilibrium. 1 temperature and thermal equilibrium when we speak of objects being \hot and \cold, we need to quantify this by some. if two systems can exchange energy, then t ∗ 1 = t ∗ 2. a thermometer measures. Mechanical Equilibrium And Thermal.
From www.youtube.com
Thermodynamics Equilibrium Thermal Equilibrium Mechanical Mechanical Equilibrium And Thermal An important concept related to temperature is thermal equilibrium. Two objects are in thermal equilibrium if. It is through the concepts of thermal equilibrium and the zeroth law of. Two objects are in thermal equilibrium if they are in close. thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the. If. Mechanical Equilibrium And Thermal.