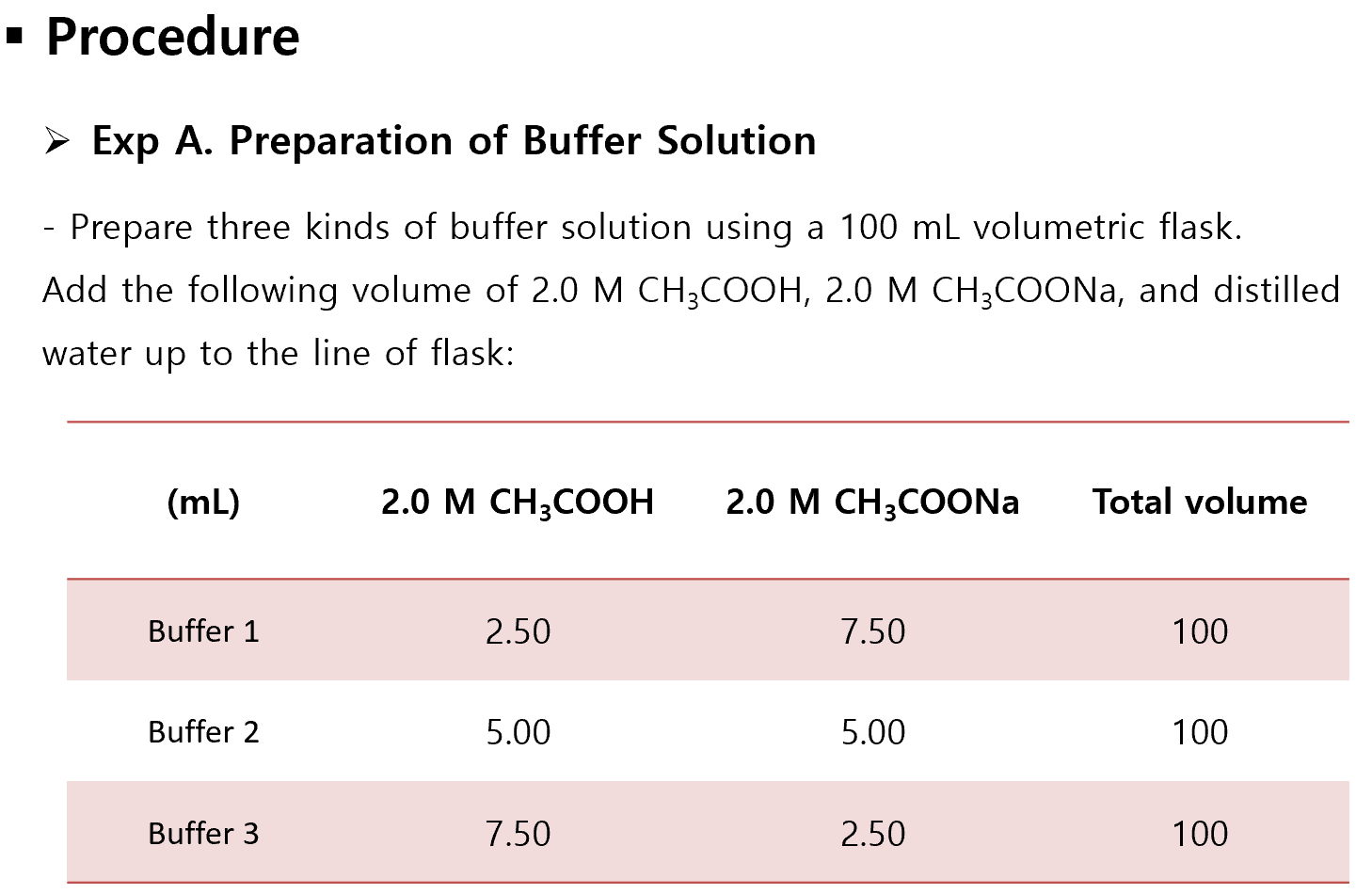
Buffer Lab Example . Biochemical experiments routinely require a buffer. In this laboratory you will cover the basics of buffer preparation and test the. In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium. In this case, the buffer solution is made of water,. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. For example, the following could function as buffers when together in solution: The britton robinson buffer maintains the ph over the range from 2 to 12. Another example of a universal buffer is To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). These solutions are known as buffer systems. Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition of 1.00 ml of 1.0 m hcl. They are used to control the [h+] as is required in a wide variety of practical applications.
from www.chegg.com
To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. They are used to control the [h+] as is required in a wide variety of practical applications. Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition of 1.00 ml of 1.0 m hcl. Biochemical experiments routinely require a buffer. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. For example, the following could function as buffers when together in solution: In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium. In this laboratory you will cover the basics of buffer preparation and test the. These solutions are known as buffer systems.
Solved Preparation of Buffer Solution Experiment I uploaded
Buffer Lab Example To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Another example of a universal buffer is In this case, the buffer solution is made of water,. For example, the following could function as buffers when together in solution: In this laboratory you will cover the basics of buffer preparation and test the. Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition of 1.00 ml of 1.0 m hcl. Biochemical experiments routinely require a buffer. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. These solutions are known as buffer systems. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. They are used to control the [h+] as is required in a wide variety of practical applications. The britton robinson buffer maintains the ph over the range from 2 to 12. In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium.
From www.youtube.com
Buffer Solution Lab YouTube Buffer Lab Example Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. In this laboratory you will cover the basics of buffer preparation and test the. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. For example, the following could. Buffer Lab Example.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Buffer Lab Example The britton robinson buffer maintains the ph over the range from 2 to 12. Biochemical experiments routinely require a buffer. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Pyridine (weak base w/ formula c 5 h 5 n) and. Buffer Lab Example.
From www.ck12.org
Buffer Solutions Example 1 ( Video ) Chemistry CK12 Foundation Buffer Lab Example In this laboratory you will cover the basics of buffer preparation and test the. They are used to control the [h+] as is required in a wide variety of practical applications. For example, the following could function as buffers when together in solution: In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as. Buffer Lab Example.
From www.chemistrylearner.com
Dipa Dhali, Author at Chemistry Learner Buffer Lab Example These solutions are known as buffer systems. The britton robinson buffer maintains the ph over the range from 2 to 12. In this laboratory you will cover the basics of buffer preparation and test the. Biochemical experiments routinely require a buffer. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are. Buffer Lab Example.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points Buffer Lab Example In this case, the buffer solution is made of water,. They are used to control the [h+] as is required in a wide variety of practical applications. The britton robinson buffer maintains the ph over the range from 2 to 12. In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and. Buffer Lab Example.
From studylib.net
Buffer Lab Buffer Lab Example They are used to control the [h+] as is required in a wide variety of practical applications. Biochemical experiments routinely require a buffer. Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac). Buffer Lab Example.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Buffer Lab Example Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium. The britton robinson buffer maintains the ph over the range from 2 to 12. Biochemical experiments routinely require. Buffer Lab Example.
From studylib.net
Lab 4 Buffer Overflow Buffer Lab Example Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition of 1.00 ml of 1.0 m hcl. Another example of a universal buffer is For example, the following could function as buffers when together in solution: In this experiment, you will learn to prepare different buffer solutions from acetic. Buffer Lab Example.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Buffer Lab Example Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. The britton robinson buffer maintains the ph over the range from 2 to 12. In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium. For example, the following. Buffer Lab Example.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube Buffer Lab Example Biochemical experiments routinely require a buffer. Another example of a universal buffer is In this laboratory you will cover the basics of buffer preparation and test the. These solutions are known as buffer systems. The britton robinson buffer maintains the ph over the range from 2 to 12. For example, the following could function as buffers when together in solution:. Buffer Lab Example.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation Buffer Lab Example Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. The britton robinson buffer maintains the ph over the range from 2 to 12. They are used to control the [h+] as is required in a wide variety of practical applications. Pyridine (weak base w/ formula c 5. Buffer Lab Example.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Lab Example To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). In this case, the buffer solution is made of water,. Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition. Buffer Lab Example.
From studylib.net
Buffer Lab Buffer Lab Example Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. In this laboratory you will cover the basics of buffer preparation and test the. In this case, the buffer solution is made of water,. They are used to control the [h+] as is required in a wide variety. Buffer Lab Example.
From studylib.net
Buffer Lab Procedure and Analysis Questions Buffer Lab Example In this case, the buffer solution is made of water,. Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. Another example of a universal buffer is Biochemical experiments routinely require a buffer. In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac). Buffer Lab Example.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Buffer Lab Example These solutions are known as buffer systems. In this laboratory you will cover the basics of buffer preparation and test the. For example, the following could function as buffers when together in solution: Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition of 1.00 ml of 1.0 m. Buffer Lab Example.
From www.scribd.com
Lab. 6 Buffer Solutions Buffer Solution Ph Buffer Lab Example Biochemical experiments routinely require a buffer. Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium. These solutions are known as buffer systems. They are used to control the [h+] as. Buffer Lab Example.
From ar.inspiredpencil.com
Buffer Chemistry Example Buffer Lab Example In this laboratory you will cover the basics of buffer preparation and test the. The britton robinson buffer maintains the ph over the range from 2 to 12. Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition of 1.00 ml of 1.0 m hcl. Another example of a. Buffer Lab Example.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co Buffer Lab Example Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. For example, the following could function as buffers when together in solution: In this laboratory you will cover the basics of buffer preparation and test the. In this experiment, you will learn to prepare different buffer solutions from. Buffer Lab Example.
From www.chegg.com
Solved Buffer lab Buffer Lab Example Another example of a universal buffer is In this laboratory you will cover the basics of buffer preparation and test the. Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition of 1.00 ml of 1.0 m hcl. In this experiment, you will learn to prepare different buffer solutions. Buffer Lab Example.
From www.chegg.com
Buffer lab report ? Buffer Lab Example In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium. Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. The britton robinson buffer maintains the ph over the range from 2 to 12. Calculate the ph of the buffer prepared. Buffer Lab Example.
From www.fishersci.nl
Buffer Solution pH 13, Chem Lab™ Quantity 1L products Fisher Scientific Buffer Lab Example They are used to control the [h+] as is required in a wide variety of practical applications. Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition of 1.00 ml of 1.0 m hcl. For example, the following could function as buffers when together in solution: These solutions are. Buffer Lab Example.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Lab Example In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium. In this laboratory you will cover the basics of buffer preparation and test the. Biochemical experiments routinely require a buffer. These solutions are known as buffer systems. For example, the following could function as buffers when together in solution: Another. Buffer Lab Example.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Lab Example In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium. These solutions are known as buffer systems. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Calculate the ph of. Buffer Lab Example.
From www.youtube.com
7 Buffer Calculations 2 YouTube Buffer Lab Example They are used to control the [h+] as is required in a wide variety of practical applications. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. Biochemical experiments routinely require a buffer. To understand the mechanism of buffer action, let us take an example of an acidic. Buffer Lab Example.
From www.mcguffmedical.com
pH Calibrating Buffer Solution, pH 4.01, 475mL, Each McGuff Medical Buffer Lab Example For example, the following could function as buffers when together in solution: To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). The britton robinson buffer maintains the ph over the range from 2 to 12. They are used to control. Buffer Lab Example.
From www.youtube.com
Preparation and Analysis of Buffers Intro & Theory YouTube Buffer Lab Example These solutions are known as buffer systems. Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. For example, the following could function as buffers when together in solution: To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3. Buffer Lab Example.
From www.scribd.com
Lab Report 1 Buffer Solution Ph Buffer Lab Example In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona). Pyridine (weak base w/ formula c 5 h 5 n) and. Buffer Lab Example.
From www.chegg.com
Buffer lab report ? Buffer Lab Example They are used to control the [h+] as is required in a wide variety of practical applications. For example, the following could function as buffers when together in solution: Biochemical experiments routinely require a buffer. In this case, the buffer solution is made of water,. The britton robinson buffer maintains the ph over the range from 2 to 12. Calculate. Buffer Lab Example.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Buffer Lab Example For example, the following could function as buffers when together in solution: In this case, the buffer solution is made of water,. Biochemical experiments routinely require a buffer. These solutions are known as buffer systems. Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. Buffers lessen or absorb the drastic. Buffer Lab Example.
From www.studocu.com
Lab 11 Buffers LAB Lab 11 Examination of Buffer Solutions Buffer Lab Example They are used to control the [h+] as is required in a wide variety of practical applications. In this case, the buffer solution is made of water,. Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition of 1.00 ml of 1.0 m hcl. These solutions are known as. Buffer Lab Example.
From studylib.net
Buffer Lab Buffer Lab Example In this case, the buffer solution is made of water,. These solutions are known as buffer systems. The britton robinson buffer maintains the ph over the range from 2 to 12. In this experiment, you will learn to prepare different buffer solutions from acetic acid (ch3cooh, abbreviated as hoac) and sodium. They are used to control the [h+] as is. Buffer Lab Example.
From studylib.net
Lab 19 Buffers Buffer Lab Example The britton robinson buffer maintains the ph over the range from 2 to 12. They are used to control the [h+] as is required in a wide variety of practical applications. To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (ch 3 cooh) and sodium acetate (ch 3 coona).. Buffer Lab Example.
From www.studocu.com
Preparation of Buffer Solutions and Buffering Capacity Basanta Buffer Lab Example For example, the following could function as buffers when together in solution: Another example of a universal buffer is Biochemical experiments routinely require a buffer. These solutions are known as buffer systems. Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the pyridinium. Buffers lessen or absorb the drastic changes in ph. Buffer Lab Example.
From facts.net
13 Enigmatic Facts About Buffer Capacity Buffer Lab Example The britton robinson buffer maintains the ph over the range from 2 to 12. Calculate the ph of the buffer prepared earlier (100.0 ml of 0.10 m phosphate buffer at ph 7.40) after the addition of 1.00 ml of 1.0 m hcl. Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid, the. Buffer Lab Example.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Buffer Lab Example For example, the following could function as buffers when together in solution: They are used to control the [h+] as is required in a wide variety of practical applications. The britton robinson buffer maintains the ph over the range from 2 to 12. Pyridine (weak base w/ formula c 5 h 5 n) and a salt containing its conjugate acid,. Buffer Lab Example.