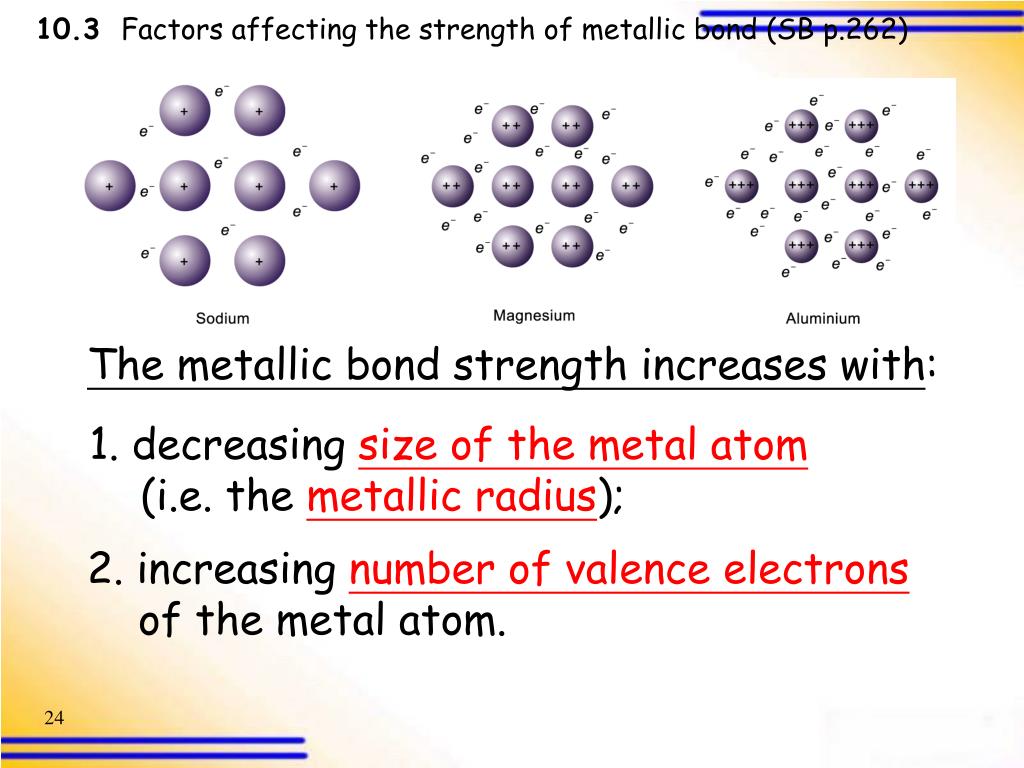
Bonding Of Metal . Metallic bonding accounts for many of the key properties of metals. Name some physical properties of metals that reflect this difference. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. This accounts for many characteristic properties of metals: metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. It can be described as the sharing of free electrons among a lattice of positively charged metal ions. A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. metallic bond, force that holds atoms together in a metallic substance. The structure of metallic bonds is entirely different from that. Ionic and covalent bonds involve only two atoms. in metallic bonding, valence electrons are delocalized or free to flow between several atoms. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. what is metallic bonding?
from www.slideserve.com
what is metallic bonding? in metallic bonding, valence electrons are delocalized or free to flow between several atoms. Name some physical properties of metals that reflect this difference. metallic bond, force that holds atoms together in a metallic substance. explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. The structure of metallic bonds is entirely different from that. This accounts for many characteristic properties of metals: Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. It can be described as the sharing of free electrons among a lattice of positively charged metal ions. metals tend to have high melting points and boiling points suggesting strong bonds between the atoms.
PPT Metallic Bonding PowerPoint Presentation, free download ID711524
Bonding Of Metal explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal. in metallic bonding, valence electrons are delocalized or free to flow between several atoms. metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. metallic bond, force that holds atoms together in a metallic substance. Ionic and covalent bonds involve only two atoms. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. what is metallic bonding? It can be described as the sharing of free electrons among a lattice of positively charged metal ions. The structure of metallic bonds is entirely different from that. Metallic bonding accounts for many of the key properties of metals. Name some physical properties of metals that reflect this difference. This accounts for many characteristic properties of metals:
From www.tec-science.com
Metallic bonding tecscience Bonding Of Metal The structure of metallic bonds is entirely different from that. Metallic bonding accounts for many of the key properties of metals. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is. Bonding Of Metal.
From www.thesciencehive.co.uk
Metallic Bonding (GCSE) — the science sauce Bonding Of Metal what is metallic bonding? The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. Metallic bonding accounts for many of the key properties of metals. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. It can be. Bonding Of Metal.
From stock.adobe.com
Metallic bond Metallic bonding is the electrostatic attractive force Bonding Of Metal This accounts for many characteristic properties of metals: Ionic and covalent bonds involve only two atoms. explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. A sheet of aluminum foil and. Bonding Of Metal.
From www.tec-science.com
Metallic bonding tecscience Bonding Of Metal in metallic bonding, valence electrons are delocalized or free to flow between several atoms. metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Metallic bonding accounts for many of the key properties of metals. It can be described as the sharing of free electrons among a lattice of positively charged metal. Bonding Of Metal.
From www.slideserve.com
PPT Unit 4 Metallic Bonding PowerPoint Presentation, free download Bonding Of Metal Ionic and covalent bonds involve only two atoms. A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The structure of metallic bonds is entirely different. Bonding Of Metal.
From www.chemistrystudent.com
Metallic Bonding (ALevel) ChemistryStudent Bonding Of Metal in metallic bonding, valence electrons are delocalized or free to flow between several atoms. The structure of metallic bonds is entirely different from that. what is metallic bonding? A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal. metals. Bonding Of Metal.
From soyungringo.blogspot.com
metallic bonding occurs between atoms of best tricktaking card games Bonding Of Metal It can be described as the sharing of free electrons among a lattice of positively charged metal ions. Metallic bonding accounts for many of the key properties of metals. The structure of metallic bonds is entirely different from that. A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to. Bonding Of Metal.
From www.slideshare.net
Metallic bonding Bonding Of Metal Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Ionic and covalent bonds involve only two atoms. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to. Bonding Of Metal.
From studyrocket.co.uk
Metallic Bonding GCSE Chemistry Science) AQA Revision Bonding Of Metal in metallic bonding, valence electrons are delocalized or free to flow between several atoms. A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal. explain the fundamental difference between the bonding in metallic solids compared to that in other types. Bonding Of Metal.
From mmerevise.co.uk
Metallic Bonding Questions and Revision MME Bonding Of Metal The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. It can be described as the sharing of free electrons among a lattice of positively charged metal ions. in metallic bonding, valence electrons are delocalized or free to flow between several atoms. what is metallic bonding? . Bonding Of Metal.
From edu.rsc.org
How to teach metallic bonding Poster RSC Education Bonding Of Metal explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The structure of metallic bonds is entirely different from that. in metallic bonding, valence electrons are delocalized or free to flow. Bonding Of Metal.
From www.pinterest.com
Metallic bonding vector illustration diagram Metallic bonding Bonding Of Metal in metallic bonding, valence electrons are delocalized or free to flow between several atoms. metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The structure of metallic bonds is entirely different from that. It can be described as the sharing of free electrons among a lattice of positively charged metal ions.. Bonding Of Metal.
From www.thoughtco.com
Definition and Properties of Metallic Bonding Bonding Of Metal metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Ionic and covalent bonds involve only two atoms. This accounts for many characteristic properties of metals: explain the fundamental difference between the. Bonding Of Metal.
From www.aakash.ac.in
Metallic Bond Definition, Examples & Properties AESL Bonding Of Metal explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. Ionic and covalent bonds involve only two atoms. what is metallic bonding? The structure of metallic. Bonding Of Metal.
From www.science-revision.co.uk
Metallic bonding Bonding Of Metal The structure of metallic bonds is entirely different from that. metallic bond, force that holds atoms together in a metallic substance. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. It can be described as the sharing of free electrons among a lattice of positively charged metal ions. The outermost electron shell. Bonding Of Metal.
From www.expii.com
Metallic Bond — Formation & Compounds Expii Bonding Of Metal The structure of metallic bonds is entirely different from that. A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. A sheet of aluminum foil and. Bonding Of Metal.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID711524 Bonding Of Metal The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. Metallic bonding accounts for many of the key properties of metals. explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. Name some physical properties of metals. Bonding Of Metal.
From www.youtube.com
Types of Bonding in Transition Metal Systems and Simple Ligands YouTube Bonding Of Metal metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. metallic bond, force that holds atoms together in a metallic substance. A metallic bond is a type of chemical bond in which a ‘cloud’ of free. Bonding Of Metal.
From www.slideserve.com
PPT Metallic bonding and properties PowerPoint Presentation, free Bonding Of Metal metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Metallic bonding accounts for many of the key properties of metals. Ionic and covalent bonds involve only two atoms. in metallic bonding, valence electrons are delocalized or free to flow between several atoms. Metals tend to have high melting points and boiling. Bonding Of Metal.
From mungfali.com
Metallic Bonding Labelled Diagram Bonding Of Metal explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. Name some physical properties of metals that reflect this difference. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. metallic bond, force that holds atoms. Bonding Of Metal.
From www.nagwa.com
Question Video Identifying the Structure of a Metallic Bond Nagwa Bonding Of Metal what is metallic bonding? The structure of metallic bonds is entirely different from that. Metallic bonding accounts for many of the key properties of metals. metallic bond, force that holds atoms together in a metallic substance. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. explain the fundamental difference between. Bonding Of Metal.
From www.slideserve.com
PPT METALLIC BOND PowerPoint Presentation, free download ID4554784 Bonding Of Metal metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Ionic and covalent bonds involve only two atoms. Metallic bonding accounts for many of the key properties of metals. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. This accounts for. Bonding Of Metal.
From www.slideserve.com
PPT Metallic Bond PowerPoint Presentation ID438230 Bonding Of Metal metallic bond, force that holds atoms together in a metallic substance. Ionic and covalent bonds involve only two atoms. The structure of metallic bonds is entirely different from that. It can be described as the sharing of free electrons among a lattice of positively charged metal ions. what is metallic bonding? explain the fundamental difference between the. Bonding Of Metal.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4982303 Bonding Of Metal A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. what is metallic bonding? Ionic and covalent bonds involve only two atoms. This accounts for. Bonding Of Metal.
From opengeology.org
2 Mineral Chemistry Mineralogy Bonding Of Metal metallic bond, force that holds atoms together in a metallic substance. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. what is metallic bonding? Metals tend to have high. Bonding Of Metal.
From mungfali.com
Metallic Bonding Labelled Diagram Bonding Of Metal A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. metallic bond, force that holds atoms together in a metallic substance. in metallic bonding, valence electrons are delocalized or free to flow between several atoms. metals tend to have high melting points and boiling points suggesting strong. Bonding Of Metal.
From blog.thepipingmart.com
Metallic Bonding in Zinc An Overview Bonding Of Metal metallic bond, force that holds atoms together in a metallic substance. Ionic and covalent bonds involve only two atoms. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. It can be described as the sharing of free electrons among a lattice of positively charged metal ions. This accounts for many characteristic properties. Bonding Of Metal.
From www.youtube.com
What is a metallic bond and how does it form Metallic Bonding Bonding Of Metal what is metallic bonding? Ionic and covalent bonds involve only two atoms. explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. This accounts for many characteristic properties of metals:. Bonding Of Metal.
From discovertutoring.co.uk
Metallic Bonding Explained Discover Tutoring Bonding Of Metal what is metallic bonding? Metallic bonding accounts for many of the key properties of metals. in metallic bonding, valence electrons are delocalized or free to flow between several atoms. A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal. A. Bonding Of Metal.
From chempedia.in
MetalLigand Bonding ChemPedia Bonding Of Metal in metallic bonding, valence electrons are delocalized or free to flow between several atoms. Metallic bonding accounts for many of the key properties of metals. This accounts for many characteristic properties of metals: Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Ionic and covalent bonds involve only two atoms. The structure. Bonding Of Metal.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID711524 Bonding Of Metal Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. metallic bond, force that holds atoms together in a metallic substance. what is metallic bonding? The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. Metallic bonding accounts for many of. Bonding Of Metal.
From www.britannica.com
chemical bonding Definition, Types, & Examples Britannica Bonding Of Metal Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. This accounts for many characteristic properties of metals: The structure of metallic bonds is entirely different from that. metallic bond, force that holds atoms together in a metallic substance. explain the fundamental difference between the bonding in metallic solids compared to that. Bonding Of Metal.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID2041377 Bonding Of Metal what is metallic bonding? The structure of metallic bonds is entirely different from that. metallic bond, force that holds atoms together in a metallic substance. It can be described as the sharing of free electrons among a lattice of positively charged metal ions. in metallic bonding, valence electrons are delocalized or free to flow between several atoms.. Bonding Of Metal.
From www.breakingatom.com
Metallic bonding Definition Bonding Of Metal The structure of metallic bonds is entirely different from that. what is metallic bonding? Name some physical properties of metals that reflect this difference. explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. Bonding Of Metal.
From www.tec-science.com
Lattice structure of metals tecscience Bonding Of Metal metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. It can be described as the sharing of free electrons among a lattice of positively charged metal ions. Metallic bonding accounts for many of the key properties of metals. Metals tend to have high melting points and boiling points suggesting strong bonds between. Bonding Of Metal.