Liquid Highest Surface Tension . since water has a high surface tension, much energy is required to separate its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The higher a liquid’s polarity, the higher the surface. In comparison, organic liquids, such as. The cohesive forces between liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. As a result of this high surface. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules.
from www.numerade.com
among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as. since water has a high surface tension, much energy is required to separate its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive forces between liquid molecules. The higher a liquid’s polarity, the higher the surface. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f).
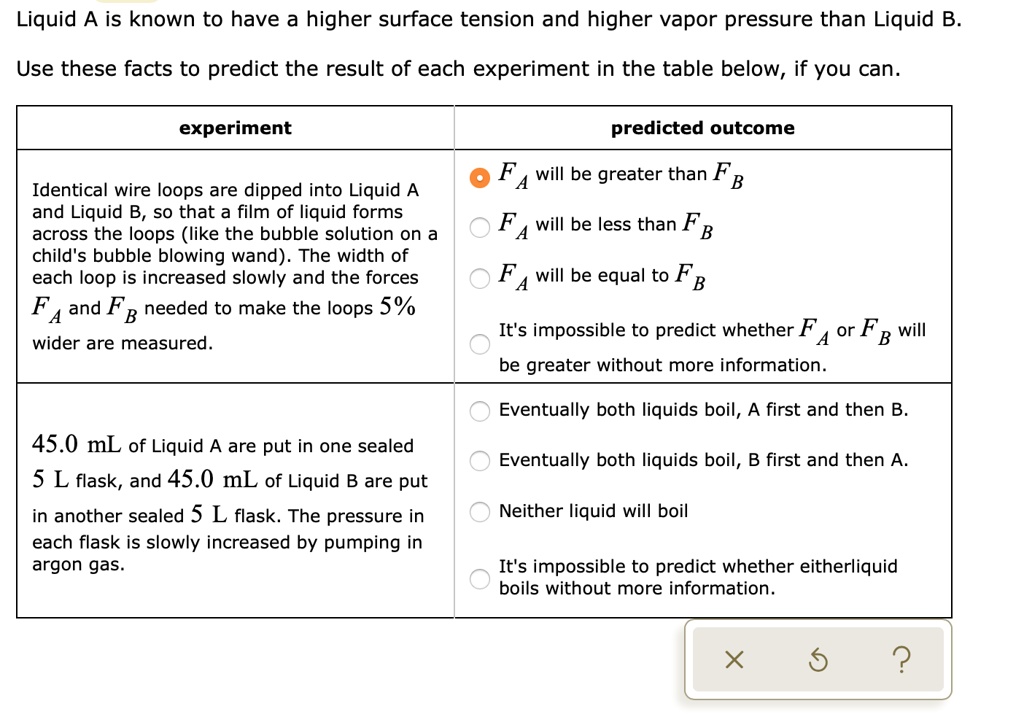
SOLVED Text Liquid A is known to have higher surface tension and
Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. since water has a high surface tension, much energy is required to separate its molecules. The cohesive forces between liquid molecules. The higher a liquid’s polarity, the higher the surface. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules.
From www.chegg.com
Solved Liquid X is known to have a higher surface tension Liquid Highest Surface Tension The higher a liquid’s polarity, the higher the surface. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules. among common liquids, water exhibits a. Liquid Highest Surface Tension.
From www.slideserve.com
PPT Properties of Liquids and Solids PowerPoint Presentation, free Liquid Highest Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules. As a result of this high surface. The higher a liquid’s polarity, the higher the surface. since water has a high surface tension, much energy is required to separate its molecules. . Liquid Highest Surface Tension.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. among common. Liquid Highest Surface Tension.
From ceelbcbq.blob.core.windows.net
Liquid Having Highest Surface Tension at Jordan Moody blog Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. . Liquid Highest Surface Tension.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download Liquid Highest Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The higher a liquid’s polarity, the higher the surface. In comparison, organic liquids, such as. As. Liquid Highest Surface Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Liquid Highest Surface Tension since water has a high surface tension, much energy is required to separate its molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. In comparison, organic liquids,. Liquid Highest Surface Tension.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with Liquid Highest Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. since water has a high surface tension, much energy is required to separate its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. water has a. Liquid Highest Surface Tension.
From www.slideserve.com
PPT Chapter 5 Liquids and Solids PowerPoint Presentation, free Liquid Highest Surface Tension As a result of this high surface. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as. since water has a high surface tension, much energy is required to separate its molecules. The higher a liquid’s polarity, the higher the surface. The. Liquid Highest Surface Tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Liquid Highest Surface Tension since water has a high surface tension, much energy is required to separate its molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The higher a liquid’s polarity, the higher the. Liquid Highest Surface Tension.
From www.numerade.com
SOLVED Which of the liquids had the highest surface tension? Paragraph Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The higher a liquid’s polarity, the higher the surface. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive forces between liquid molecules. surface tension is the energy, or. Liquid Highest Surface Tension.
From dfvswjxoeco.blob.core.windows.net
How To Determine Highest Surface Tension at Juan Patterson blog Liquid Highest Surface Tension In comparison, organic liquids, such as. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. surface tension is the energy, or work, required to increase the surface area of a. Liquid Highest Surface Tension.
From www.biolinscientific.com
Why is surface tension important? Liquid Highest Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules. since water has a high surface tension, much energy is required to separate its molecules. In comparison, organic liquids, such as. The higher a liquid’s polarity, the higher the surface. among. Liquid Highest Surface Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. since water has a high surface tension, much energy is required to separate its molecules. water has a surface tension. Liquid Highest Surface Tension.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The higher a liquid’s polarity, the higher the surface. In comparison, organic liquids, such as. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The cohesive forces between liquid molecules. surface tension. Liquid Highest Surface Tension.
From www.youtube.com
What is Surface Tension Liquid State Liquid Dynamics Surface Liquid Highest Surface Tension The higher a liquid’s polarity, the higher the surface. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. water has a surface tension. Liquid Highest Surface Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Liquid Highest Surface Tension As a result of this high surface. The higher a liquid’s polarity, the higher the surface. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between. Liquid Highest Surface Tension.
From www.biolinscientific.com
Surface tension of water Why is it so high? Liquid Highest Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. . Liquid Highest Surface Tension.
From chemwiki.ucdavis.edu
11.3 Unique Properties of Liquids Chemwiki Liquid Highest Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). since water has a high surface tension, much energy is required to separate its molecules. The higher a liquid’s polarity, the higher the surface. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its. Liquid Highest Surface Tension.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b Liquid Highest Surface Tension In comparison, organic liquids, such as. since water has a high surface tension, much energy is required to separate its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The higher a liquid’s polarity, the higher the surface. among common liquids, water exhibits a distinctly high surface. Liquid Highest Surface Tension.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Liquid Highest Surface Tension As a result of this high surface. The cohesive forces between liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. since water has a high surface tension, much energy is required to separate its molecules. water has a surface tension of 0.07275 joule per. Liquid Highest Surface Tension.
From byjus.com
Explain the surface tension phenomenon with examples. Liquid Highest Surface Tension since water has a high surface tension, much energy is required to separate its molecules. In comparison, organic liquids, such as. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The higher a liquid’s polarity, the higher the surface. among common liquids, water exhibits a distinctly high surface tension due. Liquid Highest Surface Tension.
From ceelbcbq.blob.core.windows.net
Liquid Having Highest Surface Tension at Jordan Moody blog Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The higher a liquid’s polarity, the higher the surface. since water has a high surface tension, much energy is required to separate its molecules. surface tension is the energy, or work, required to increase the surface area of a. Liquid Highest Surface Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. In comparison, organic liquids, such as. since water has a high surface tension, much energy is required to separate its molecules. The cohesive forces between liquid molecules. water has a surface tension of 0.07275 joule per square metre at. Liquid Highest Surface Tension.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Liquid Highest Surface Tension The higher a liquid’s polarity, the higher the surface. since water has a high surface tension, much energy is required to separate its molecules. In comparison, organic liquids, such as. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface. water has. Liquid Highest Surface Tension.
From cesioues.blob.core.windows.net
High Surface Tension Facts at Margaret Halpern blog Liquid Highest Surface Tension since water has a high surface tension, much energy is required to separate its molecules. As a result of this high surface. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The higher a liquid’s polarity, the higher the surface. water has a surface tension of 0.07275 joule. Liquid Highest Surface Tension.
From cevwtnpi.blob.core.windows.net
Liquid Drop And Surface Tension at Stacey Craver blog Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. In comparison, organic liquids, such as. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. among common liquids, water exhibits a distinctly high surface tension due to strong. Liquid Highest Surface Tension.
From cevwtnpi.blob.core.windows.net
Liquid Drop And Surface Tension at Stacey Craver blog Liquid Highest Surface Tension since water has a high surface tension, much energy is required to separate its molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. As a result of this high surface. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding. Liquid Highest Surface Tension.
From www.gauthmath.com
Solved Which liquid would have the highest surface tension (ability to Liquid Highest Surface Tension In comparison, organic liquids, such as. The cohesive forces between liquid molecules. since water has a high surface tension, much energy is required to separate its molecules. The higher a liquid’s polarity, the higher the surface. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water. Liquid Highest Surface Tension.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Liquid Highest Surface Tension The higher a liquid’s polarity, the higher the surface. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. since water has a high surface tension, much energy is required to separate its molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68. Liquid Highest Surface Tension.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Liquid Highest Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The. Liquid Highest Surface Tension.
From www.chegg.com
Solved Liquid A is known to have a higher surface tension Liquid Highest Surface Tension since water has a high surface tension, much energy is required to separate its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. surface tension is the energy,. Liquid Highest Surface Tension.
From ceelbcbq.blob.core.windows.net
Liquid Having Highest Surface Tension at Jordan Moody blog Liquid Highest Surface Tension The higher a liquid’s polarity, the higher the surface. In comparison, organic liquids, such as. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. among common liquids, water exhibits a distinctly high. Liquid Highest Surface Tension.
From www.numerade.com
SOLVED Text Liquid A is known to have higher surface tension and Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. In comparison, organic liquids, such as. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. among common liquids, water exhibits a distinctly high surface tension due to strong. Liquid Highest Surface Tension.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Liquid Highest Surface Tension The cohesive forces between liquid molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface. In comparison, organic liquids, such as. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). since water has a. Liquid Highest Surface Tension.
From www.thoughtco.com
Surface Tension Definition in Chemistry Liquid Highest Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The cohesive forces between liquid molecules. among common liquids, water exhibits a distinctly high surface tension. Liquid Highest Surface Tension.