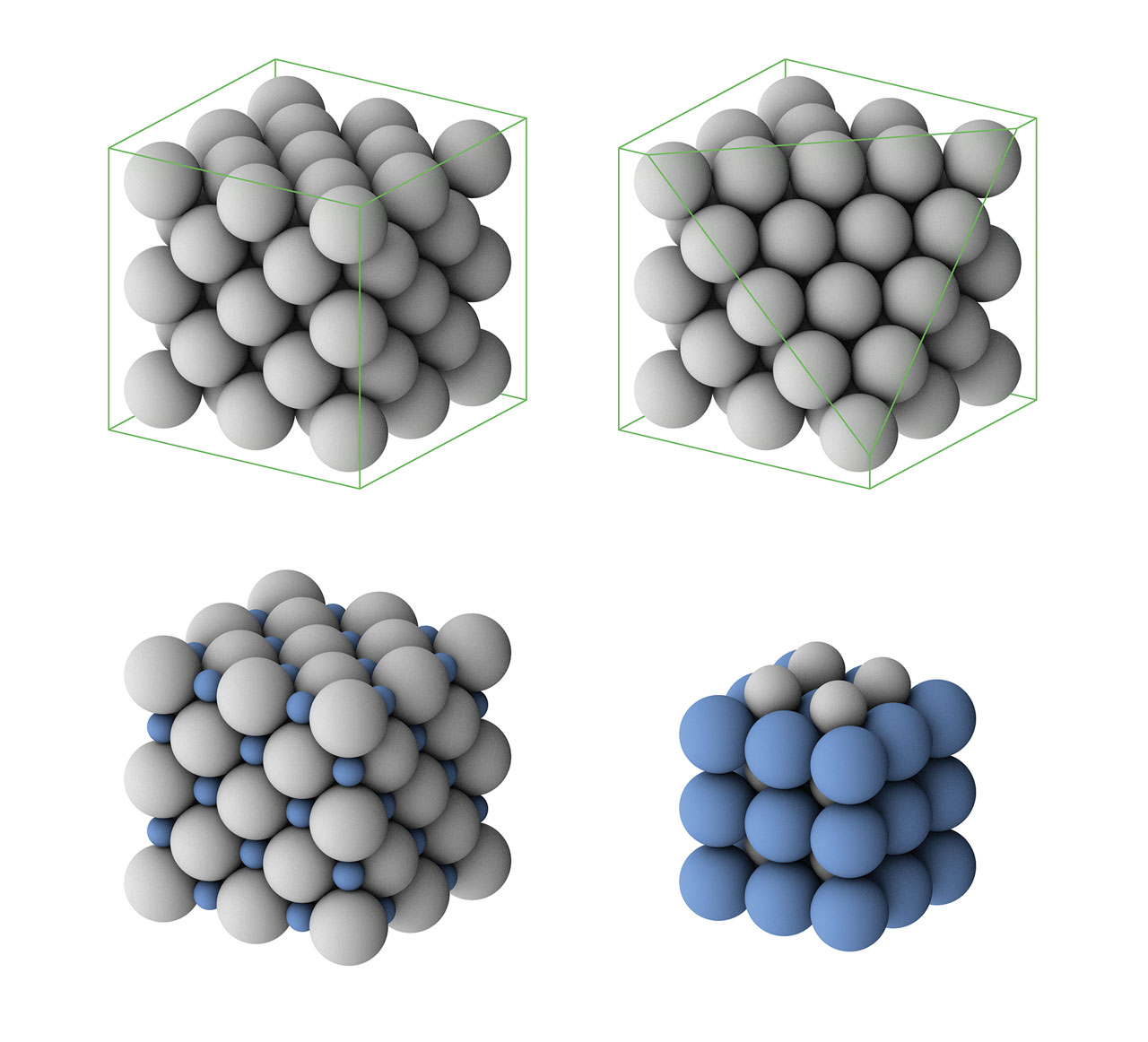
Chemistry Structure Glass . Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. The main constituent of flat glass is sio 2 (silica sand). glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. This has a high melting temperature in the region of 1700 degrees c and its. Natural forms of silicate glass also exist. presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase.
from www.beautifulchemistry.net
presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. Natural forms of silicate glass also exist. Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. The main constituent of flat glass is sio 2 (silica sand). some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. This has a high melting temperature in the region of 1700 degrees c and its. glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to.
Crystal Structure — Beautiful Chemistry
Chemistry Structure Glass presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. This has a high melting temperature in the region of 1700 degrees c and its. glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. Natural forms of silicate glass also exist. The main constituent of flat glass is sio 2 (silica sand). compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass.
From www.researchgate.net
Schematic illustrations of glass structures The structures for parent Chemistry Structure Glass glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. This has a high melting temperature in the region of 1700 degrees c and its. presenting the fundamental topics in. Chemistry Structure Glass.
From chem.libretexts.org
11.7 Structure of Solids Chemistry LibreTexts Chemistry Structure Glass The main constituent of flat glass is sio 2 (silica sand). Natural forms of silicate glass also exist. glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. This has a high. Chemistry Structure Glass.
From www.dreamstime.com
Chemical Structure Written on Glass Stock Image Image of bonds Chemistry Structure Glass glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. Natural forms of silicate glass also exist. Impurities or additional elements and compounds added to the silicate change the color and other properties. Chemistry Structure Glass.
From www.cmog.org
All About Glass Corning Museum of Glass Chemistry Structure Glass This has a high melting temperature in the region of 1700 degrees c and its. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. Natural forms of silicate glass also exist. presenting the. Chemistry Structure Glass.
From www.slideserve.com
PPT COMMERCIAL FIBERS PowerPoint Presentation, free download ID6737083 Chemistry Structure Glass compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. The main constituent of flat glass is sio 2 (silica sand). presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. Natural forms of silicate glass also exist. some liquids, because of complex. Chemistry Structure Glass.
From opengeology.org
13 Crystal Structures Mineralogy Chemistry Structure Glass Natural forms of silicate glass also exist. The main constituent of flat glass is sio 2 (silica sand). This has a high melting temperature in the region of 1700 degrees c and its. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. compare crystalline and amorphous solids in terms of. Chemistry Structure Glass.
From www.pinterest.com
Ideal Glass Would Explain Why Glass Exists at All Quanta Magazine Chemistry Structure Glass The main constituent of flat glass is sio 2 (silica sand). Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. some liquids, because of complex molecular configuration or slow molecular transport, do. Chemistry Structure Glass.
From anaylock.blogspot.com
Common Glass Chemical Formula Ion Names, Formulas and Charges Chart Chemistry Structure Glass The main constituent of flat glass is sio 2 (silica sand). Natural forms of silicate glass also exist. presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. This has a high melting. Chemistry Structure Glass.
From techiescientist.com
Does Glass Conduct Heat? Techiescientist Chemistry Structure Glass Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. Natural forms of silicate glass. Chemistry Structure Glass.
From vitrumlife.it
La chimie du verre structure, avantages et tests de résistance Chemistry Structure Glass glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. The main constituent of flat glass is sio 2 (silica sand). This has a high melting temperature in the region of 1700 degrees c and its. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic. Chemistry Structure Glass.
From www.researchgate.net
An infinite element K. Download Scientific Diagram Chemistry Structure Glass Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. The main constituent of. Chemistry Structure Glass.
From researchblog.duke.edu
Physics Archives Page 9 of 10 Research Blog Chemistry Structure Glass compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. The main constituent of flat glass is sio 2 (silica sand). glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. presenting the fundamental topics in glass science and technology, this concise. Chemistry Structure Glass.
From www.nature.com
Atomic structure of a glass imaged at last Chemistry Structure Glass compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. The main constituent of flat glass is sio 2 (silica sand). glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. This has a high melting temperature in the region of 1700 degrees. Chemistry Structure Glass.
From www.alamy.com
Acrylic glass or poly(methyl methacrylate), chemical structure. PMMA is Chemistry Structure Glass Natural forms of silicate glass also exist. presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize”. Chemistry Structure Glass.
From www.alamy.com
Abstract molecular structure with glass material. Isolated on white Chemistry Structure Glass some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. The main constituent of flat glass is sio 2 (silica sand). Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. presenting the fundamental topics in glass science and technology, this concise. Chemistry Structure Glass.
From www.slideserve.com
PPT Ch 4 Physical Properties Glass and Soil PowerPoint Chemistry Structure Glass Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. This has a high melting temperature in the region of 1700 degrees c and its. Natural forms of silicate glass also exist. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. The main. Chemistry Structure Glass.
From www.e-education.psu.edu
Silica (SiO2) MATSE 81 Materials In Today's World Chemistry Structure Glass compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. The main constituent of flat glass is sio 2 (silica sand). Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. presenting the fundamental topics in glass science and technology, this concise introduction. Chemistry Structure Glass.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Chemistry Structure Glass Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. Natural forms of silicate glass also exist. This has a high melting temperature in the region of 1700 degrees c and its. . Chemistry Structure Glass.
From www.dreamstime.com
Acrylic Glass (pmma, Poly(methyl Methacrylate) ), Chemical Structure Chemistry Structure Glass Natural forms of silicate glass also exist. Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. some liquids, because of complex molecular configuration or slow molecular transport, do not. Chemistry Structure Glass.
From www.beautifulchemistry.net
Crystal Structure — Beautiful Chemistry Chemistry Structure Glass compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. The main constituent of. Chemistry Structure Glass.
From newsroom.ucla.edu
Photo Atomic structure glass UCLA Chemistry Structure Glass Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. The main constituent of flat glass is sio 2 (silica sand). This has a high melting temperature in the region of. Chemistry Structure Glass.
From mungfali.com
Atomic Structure Of Glass Chemistry Structure Glass Natural forms of silicate glass also exist. presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. The main constituent of flat glass is sio 2 (silica sand). some liquids, because of complex. Chemistry Structure Glass.
From www.researchgate.net
Sodalime glass chemical structure Download Scientific Diagram Chemistry Structure Glass presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. Natural forms of silicate glass also exist. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition. Chemistry Structure Glass.
From www.researchgate.net
For each of the amorphous glass structures with the crystalline Chemistry Structure Glass compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. This has a high melting temperature in the region of 1700 degrees c and its. Natural forms of silicate glass also exist. . Chemistry Structure Glass.
From www.visionlearning.com
Propiedades de Solidos Chemistry Visionlearning Chemistry Structure Glass glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. Natural forms of silicate glass also exist. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. This has a high melting temperature in the region of 1700 degrees c and its.. Chemistry Structure Glass.
From cartoondealer.com
Glass Molecules On A Chemistry Chart RoyaltyFree Stock Photo Chemistry Structure Glass presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. This has. Chemistry Structure Glass.
From www.dreamstime.com
Fullerene Chemical Science Structure with Flask Glass Equipment Stock Chemistry Structure Glass This has a high melting temperature in the region of 1700 degrees c and its. The main constituent of flat glass is sio 2 (silica sand). Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure,. Chemistry Structure Glass.
From www.dreamstime.com
Glass Molecule Structure Visualization Stock Illustration Chemistry Structure Glass some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. glass, an inorganic solid material that is usually transparent or translucent as well as hard, brittle, and impervious to. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. The main constituent of flat. Chemistry Structure Glass.
From asdn.net
ASDN Chemistry Silicates Chemistry Structure Glass This has a high melting temperature in the region of 1700 degrees c and its. Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. The main constituent of flat glass is sio. Chemistry Structure Glass.
From chemistry.stackexchange.com
chemistry Shapes of ionic compound Chemistry Stack Exchange Chemistry Structure Glass some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. Natural forms of silicate glass also exist. compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition. Chemistry Structure Glass.
From courses.lumenlearning.com
Properties of Quartz and Glass Introduction to Chemistry Chemistry Structure Glass The main constituent of flat glass is sio 2 (silica sand). compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. This has a high melting temperature in the region of 1700 degrees c and its. Natural forms of silicate glass also exist. glass, an inorganic solid material that is usually transparent. Chemistry Structure Glass.
From mungfali.com
Glass Crystal Structure Chemistry Structure Glass Natural forms of silicate glass also exist. Impurities or additional elements and compounds added to the silicate change the color and other properties of the glass. The main constituent of flat glass is sio 2 (silica sand). This has a high melting temperature in the region of 1700 degrees c and its. glass, an inorganic solid material that is. Chemistry Structure Glass.
From julianailkemp.blogspot.com
Common Glass Chemical Formula JulianailKemp Chemistry Structure Glass some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. The main constituent of flat glass is sio 2 (silica sand). Impurities or additional elements and compounds added to the silicate change the. Chemistry Structure Glass.
From www.britannica.com
crystal Types of bonds Britannica Chemistry Structure Glass The main constituent of flat glass is sio 2 (silica sand). some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. This has a high melting temperature in the region of 1700 degrees c and its. Impurities or additional elements and compounds added to the silicate change the color and other properties. Chemistry Structure Glass.
From emoryinsiena2012.blogspot.com
Chemistry Studies in Siena 2012 Lead Oxide Exploring the difference Chemistry Structure Glass compare crystalline and amorphous solids in terms of their composition, molar volume, atomic structure, transition temperature,. This has a high melting temperature in the region of 1700 degrees c and its. Natural forms of silicate glass also exist. presenting the fundamental topics in glass science and technology, this concise introduction includes glass formation, crystallization, and phase. glass,. Chemistry Structure Glass.