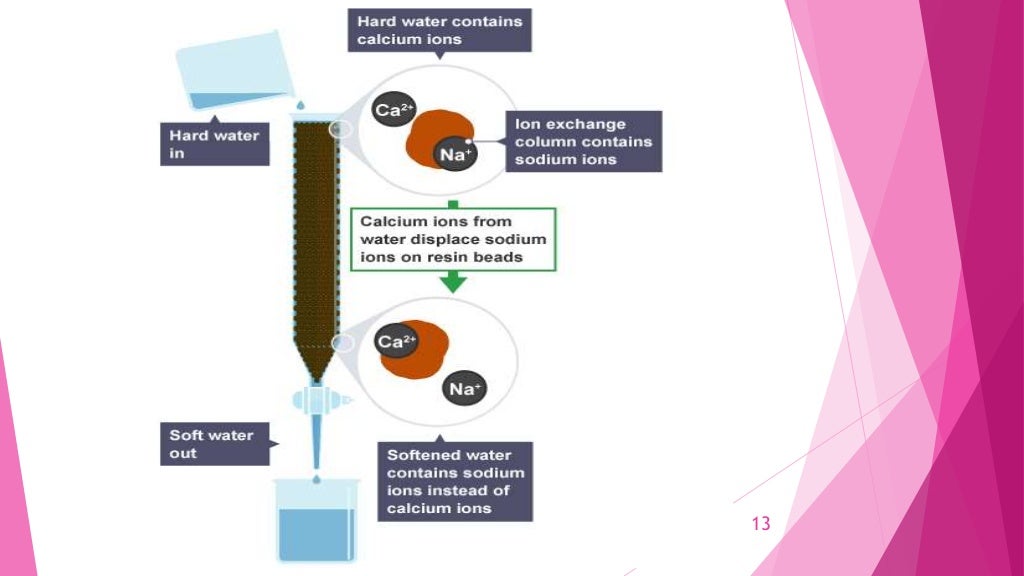
Ion Exchange Chromatography Khan Academy . what is ion exchange chromatography? understand the basic principles of different kinds of chromatography: Ion exchange chromatography (or ion chromatography) is a. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). This technique is used to analyze ionic substances. in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. the basic process of chromatography using ion exchange can be represented in 5 steps:
from www.slideshare.net
Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. Ion exchange chromatography (or ion chromatography) is a. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). the basic process of chromatography using ion exchange can be represented in 5 steps: what is ion exchange chromatography? in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. understand the basic principles of different kinds of chromatography: This technique is used to analyze ionic substances.
Ion Exchange Chromatography ppt
Ion Exchange Chromatography Khan Academy what is ion exchange chromatography? in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. what is ion exchange chromatography? This technique is used to analyze ionic substances. the basic process of chromatography using ion exchange can be represented in 5 steps: understand the basic principles of different kinds of chromatography: Ion exchange chromatography (or ion chromatography) is a. Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium).
From www.slideserve.com
PPT Ion Exchange Chromatography PowerPoint Presentation, free Ion Exchange Chromatography Khan Academy This technique is used to analyze ionic substances. what is ion exchange chromatography? in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. the basic process of chromatography using ion exchange can be represented in 5 steps: Eluent loading, sample injection, separation of sample, elution of analyte a, and. Ion Exchange Chromatography Khan Academy.
From chemistrywithwiley.com
Ion Exchange Chromatography Types of ion exchangers Factors Ion Exchange Chromatography Khan Academy what is ion exchange chromatography? It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). understand the basic principles of different kinds of chromatography: Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. in ion exchange. Ion Exchange Chromatography Khan Academy.
From www.studocu.com
Ion Exchange Chromatography The principle of separation is thus by Ion Exchange Chromatography Khan Academy what is ion exchange chromatography? Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). Ion exchange chromatography (or ion chromatography) is a. in ion exchange chromatography, the. Ion Exchange Chromatography Khan Academy.
From www.researchgate.net
Basic scheme of ionexchange chromatography Download Scientific Diagram Ion Exchange Chromatography Khan Academy Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. This technique is used to analyze ionic substances. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). Ion exchange chromatography (or ion chromatography) is a. understand the basic. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Introduction to Ion Exchange Chromatography (IEX) YouTube Ion Exchange Chromatography Khan Academy the basic process of chromatography using ion exchange can be represented in 5 steps: what is ion exchange chromatography? in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium).. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Ion Exchange Chromatography YouTube Ion Exchange Chromatography Khan Academy in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). Ion exchange chromatography (or ion chromatography) is a. understand the basic principles of different kinds of chromatography: the basic. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Ion Exchange Chromatography Theory and Principle YouTube Ion Exchange Chromatography Khan Academy Ion exchange chromatography (or ion chromatography) is a. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). the basic process of chromatography using ion exchange can be represented in 5 steps: understand the basic principles of different kinds of chromatography: in ion exchange chromatography, the support. Ion Exchange Chromatography Khan Academy.
From www.researchgate.net
Schematic illustration of ionexchange chromatography. The Ion Exchange Chromatography Khan Academy This technique is used to analyze ionic substances. in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. understand the basic principles of different kinds of chromatography: Ion exchange chromatography (or ion chromatography) is a. what is ion exchange chromatography? It is often used for inorganic anions (e.g., chloride,. Ion Exchange Chromatography Khan Academy.
From scienceinfo.com
Ion Exchange Chromatography Principle, Types, Procedure, Applications Ion Exchange Chromatography Khan Academy It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). the basic process of chromatography using ion exchange can be represented in 5 steps: understand the basic principles of different kinds of chromatography: Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte. Ion Exchange Chromatography Khan Academy.
From conductscience.com
Ionexchange Chromatography Protocol Conduct Science Ion Exchange Chromatography Khan Academy This technique is used to analyze ionic substances. what is ion exchange chromatography? in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. It is often used for inorganic anions. Ion Exchange Chromatography Khan Academy.
From microbenotes.com
Ion Exchange Chromatography Principle, Parts, Steps, Uses Ion Exchange Chromatography Khan Academy the basic process of chromatography using ion exchange can be represented in 5 steps: It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). understand the basic principles of different kinds of chromatography: in ion exchange chromatography, the support consists of tiny beads to which are attached. Ion Exchange Chromatography Khan Academy.
From www.intechopen.com
Ion Exchange Chromatography An Overview IntechOpen Ion Exchange Chromatography Khan Academy in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. Ion exchange chromatography (or ion chromatography) is a. what is ion exchange chromatography? It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). Eluent loading, sample injection, separation of sample,. Ion Exchange Chromatography Khan Academy.
From chemistnotes.com
Ion Exchange Chromatography,Defination, principle and advantages Ion Exchange Chromatography Khan Academy Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. Ion exchange chromatography (or ion chromatography) is a. understand the basic principles of different kinds of chromatography: what is ion exchange chromatography? It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g.,. Ion Exchange Chromatography Khan Academy.
From www.intechopen.com
IonExchange Chromatography and Its Applications IntechOpen Ion Exchange Chromatography Khan Academy It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). understand the basic principles of different kinds of chromatography: the basic process of chromatography using ion exchange can be represented in 5 steps: This technique is used to analyze ionic substances. Ion exchange chromatography (or ion chromatography) is. Ion Exchange Chromatography Khan Academy.
From www.intechopen.com
Ion Exchange Chromatography An Overview IntechOpen Ion Exchange Chromatography Khan Academy This technique is used to analyze ionic substances. Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). what is ion exchange chromatography? Ion exchange chromatography (or ion chromatography). Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Ion exchange chromatography YouTube Ion Exchange Chromatography Khan Academy It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). the basic process of chromatography using ion exchange can be represented in 5 steps: Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. Ion exchange chromatography (or ion. Ion Exchange Chromatography Khan Academy.
From microbenotes.com
Ion Exchange Chromatography Principle, Parts, Steps, Uses Ion Exchange Chromatography Khan Academy This technique is used to analyze ionic substances. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. what is ion exchange chromatography? the basic process of chromatography using. Ion Exchange Chromatography Khan Academy.
From biochemden.com
What is Ion Exchange Chromatography and its Applications Ion Exchange Chromatography Khan Academy Ion exchange chromatography (or ion chromatography) is a. understand the basic principles of different kinds of chromatography: the basic process of chromatography using ion exchange can be represented in 5 steps: It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). what is ion exchange chromatography? This. Ion Exchange Chromatography Khan Academy.
From microbiologynotes.org
Ion Exchange chromatography Principle, Technique and Application Ion Exchange Chromatography Khan Academy It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). This technique is used to analyze ionic substances. understand the basic principles of different kinds of chromatography: in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. the basic. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Chromatography Middle school physics Khan Academy YouTube Ion Exchange Chromatography Khan Academy Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). Ion. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Ion Exchange Chromatography Animation YouTube Ion Exchange Chromatography Khan Academy understand the basic principles of different kinds of chromatography: This technique is used to analyze ionic substances. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. the. Ion Exchange Chromatography Khan Academy.
From www.slideserve.com
PPT Ion exchange chromatography PowerPoint Presentation, free Ion Exchange Chromatography Khan Academy understand the basic principles of different kinds of chromatography: Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. Ion exchange chromatography (or ion chromatography) is a. the basic. Ion Exchange Chromatography Khan Academy.
From thechemistrynotes.com
Ion Exchange Chromatography Principle, Types, Procedure, Applications Ion Exchange Chromatography Khan Academy It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). what is ion exchange chromatography? in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte. Ion Exchange Chromatography Khan Academy.
From www.studypool.com
SOLUTION Ion exchange chromatography Studypool Ion Exchange Chromatography Khan Academy Ion exchange chromatography (or ion chromatography) is a. in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. the basic process of chromatography using ion exchange can be represented in 5 steps: what is ion exchange chromatography? Eluent loading, sample injection, separation of sample, elution of analyte a, and. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Principles of Ion exchange chromatography YouTube Ion Exchange Chromatography Khan Academy Ion exchange chromatography (or ion chromatography) is a. in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. This technique is used to analyze ionic substances. Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. understand the basic principles. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
The Principle Of Ion Exchange Chromatography, A Full Explanation YouTube Ion Exchange Chromatography Khan Academy Ion exchange chromatography (or ion chromatography) is a. the basic process of chromatography using ion exchange can be represented in 5 steps: Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. This technique is used to analyze ionic substances. understand the basic principles of different kinds of. Ion Exchange Chromatography Khan Academy.
From www.intechopen.com
IonExchange Chromatography and Its Applications IntechOpen Ion Exchange Chromatography Khan Academy what is ion exchange chromatography? the basic process of chromatography using ion exchange can be represented in 5 steps: Ion exchange chromatography (or ion chromatography) is a. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). in ion exchange chromatography, the support consists of tiny beads. Ion Exchange Chromatography Khan Academy.
From medium.com
Applications of ion exchange chromatography Study Chemistry Ion Exchange Chromatography Khan Academy in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. what is ion exchange chromatography? the basic process of chromatography using ion exchange can be represented in 5 steps: It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium).. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Ion Exchange Chromatography Principle of ion Exchange Chromatography Ion Exchange Chromatography Khan Academy what is ion exchange chromatography? the basic process of chromatography using ion exchange can be represented in 5 steps: in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium).. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Ion exchange chromatography YouTube Ion Exchange Chromatography Khan Academy understand the basic principles of different kinds of chromatography: This technique is used to analyze ionic substances. Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. the basic process of chromatography using ion exchange can be represented in 5 steps: Ion exchange chromatography (or ion chromatography) is. Ion Exchange Chromatography Khan Academy.
From www.slideshare.net
Ion Exchange Chromatography ppt Ion Exchange Chromatography Khan Academy Ion exchange chromatography (or ion chromatography) is a. Eluent loading, sample injection, separation of sample, elution of analyte a, and elution of analyte b, shown and explained below. what is ion exchange chromatography? understand the basic principles of different kinds of chromatography: the basic process of chromatography using ion exchange can be represented in 5 steps: This. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Ion chromatography (Ion pair and Ion Exchange chromatography) basic Ion Exchange Chromatography Khan Academy It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). what is ion exchange chromatography? the basic process of chromatography using ion exchange can be represented in 5 steps: Ion exchange chromatography (or ion chromatography) is a. understand the basic principles of different kinds of chromatography: Eluent. Ion Exchange Chromatography Khan Academy.
From www.researchgate.net
Schematic illustration of ionexchange chromatography. The Ion Exchange Chromatography Khan Academy what is ion exchange chromatography? in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. This technique is used to analyze ionic substances. the basic process of chromatography using ion exchange can be represented in 5 steps: Ion exchange chromatography (or ion chromatography) is a. Eluent loading, sample injection,. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Ion Exchange Chromatography Theory & Principle, Factors affecting Ion Exchange Chromatography Khan Academy It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). in ion exchange chromatography, the support consists of tiny beads to which are attached chemicals possessing a charge. This technique is used to analyze ionic substances. Eluent loading, sample injection, separation of sample, elution of analyte a, and elution. Ion Exchange Chromatography Khan Academy.
From www.youtube.com
Ion exchange chromatography YouTube Ion Exchange Chromatography Khan Academy understand the basic principles of different kinds of chromatography: It is often used for inorganic anions (e.g., chloride, nitrate, and sulfate) and inorganic cations (e.g., lithium, sodium, and potassium). the basic process of chromatography using ion exchange can be represented in 5 steps: Ion exchange chromatography (or ion chromatography) is a. Eluent loading, sample injection, separation of sample,. Ion Exchange Chromatography Khan Academy.