Are Ideal Gases Diatomic . As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. A diatomic ideal gas equation: The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. Such a gas has more degrees of freedom than a monatomic gas.
from www.doubtnut.com
\ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). Such a gas has more degrees of freedom than a monatomic gas. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. A diatomic ideal gas equation: As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,.
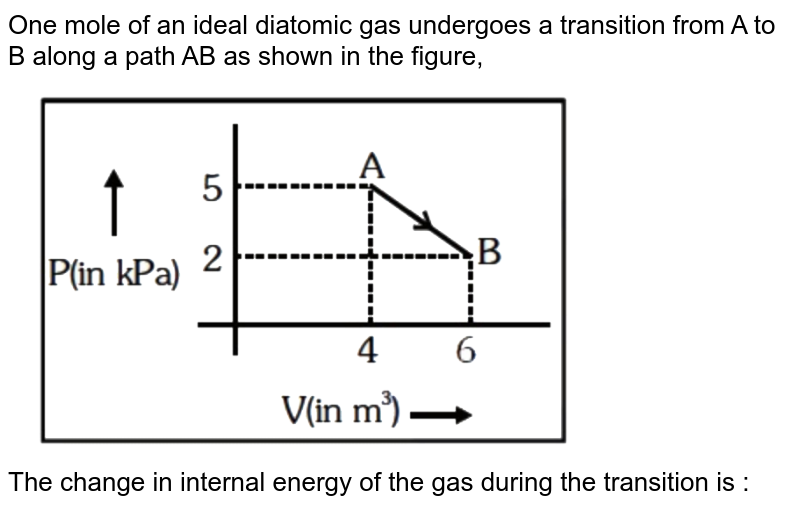
One mole of an ideal diatomic gas undergoes a transition from A to
Are Ideal Gases Diatomic \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. A diatomic ideal gas equation: Such a gas has more degrees of freedom than a monatomic gas. For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the.
From www.doubtnut.com
One mole of an ideal diatomic gas undergoes a transition from A to Are Ideal Gases Diatomic The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. Such a gas has more degrees of freedom than a monatomic gas.. Are Ideal Gases Diatomic.
From byjus.com
for two mole of diatomic gas work done at constant pressure is w. the Are Ideal Gases Diatomic A diatomic ideal gas equation: For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three. Are Ideal Gases Diatomic.
From askfilo.com
An ideal diatomic gas is carried around the cycle ABCDA as shown in figur.. Are Ideal Gases Diatomic The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). Such a gas has more degrees of freedom than a monatomic gas. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a. Are Ideal Gases Diatomic.
From www.youtube.com
Internal Energy of an Ideal Gas Molar Heat Capacity of Monatomic Are Ideal Gases Diatomic The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. For example, consider a diatomic ideal gas (a good model for nitrogen,. Are Ideal Gases Diatomic.
From byjus.com
One mole of an ideal gas whose pressure changes with volume as P=αV Are Ideal Gases Diatomic \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. Such a gas has more degrees of freedom than a monatomic gas. The. Are Ideal Gases Diatomic.
From www.toppr.com
An ideal diatomic, gas undergoes a polytropic process described by the Are Ideal Gases Diatomic \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. For example,. Are Ideal Gases Diatomic.
From www.youtube.com
3. 11P13.2 CV 2 Specific Heat Capacities of Gases and Mean Free Path Are Ideal Gases Diatomic For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). Such a gas has more degrees of freedom than a monatomic gas. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^. Are Ideal Gases Diatomic.
From askfilo.com
One mole of a diatomic ideal gas undergoes a process shown in P−V diagram.. Are Ideal Gases Diatomic As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. Such a gas has more degrees of freedom than a monatomic gas. \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. The. Are Ideal Gases Diatomic.
From www.youtube.com
1.3 Ideal gas equation YouTube Are Ideal Gases Diatomic Such a gas has more degrees of freedom than a monatomic gas. As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. For example, consider a diatomic ideal gas (a good model for nitrogen,. Are Ideal Gases Diatomic.
From kunduz.com
[ANSWERED] A diatomic ideal gas initially at temperature T is enclosed Are Ideal Gases Diatomic A diatomic ideal gas equation: For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. As per the kinetic theory of ideal gases, ideal gases are considered to. Are Ideal Gases Diatomic.
From askfilo.com
Theory 10. Consider two ideal diatomic gases A and B at some temp.. Are Ideal Gases Diatomic A diatomic ideal gas equation: As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. Such a gas. Are Ideal Gases Diatomic.
From www.youtube.com
Adiabatic Expansion of Diatomic Ideal Gas Example YouTube Are Ideal Gases Diatomic The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. Such a gas has more degrees of freedom than a monatomic gas. For example, consider a diatomic. Are Ideal Gases Diatomic.
From www.toppr.com
A 2.00mol sample of a diatomic ideal gas expands slowly and Are Ideal Gases Diatomic A diatomic ideal gas equation: \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. Such a gas has more degrees of freedom. Are Ideal Gases Diatomic.
From www.youtube.com
The internal energy of an ideal diatomic gas corresponding to volume V Are Ideal Gases Diatomic For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). A diatomic ideal gas equation: As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. \ [ z. Are Ideal Gases Diatomic.
From www.toppr.com
Consider two ideal diatomic gases A and B at some temperature T Are Ideal Gases Diatomic \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. A diatomic ideal gas equation: As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). Such a gas. Are Ideal Gases Diatomic.
From www.slideserve.com
PPT Chapter 19 PowerPoint Presentation, free download ID3073897 Are Ideal Gases Diatomic For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. Such a gas has more degrees of freedom than a monatomic gas. A diatomic ideal gas equation: \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^. Are Ideal Gases Diatomic.
From www.toppr.com
The internal energy of an ideal diatomic gas corresponding to volume V Are Ideal Gases Diatomic \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. A diatomic ideal gas equation: Such a gas has more degrees of freedom than a monatomic gas. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can. Are Ideal Gases Diatomic.
From askfilo.com
18. An ideal diatomic gas undergoes a cyclic process as shown in P−V diag.. Are Ideal Gases Diatomic \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. A diatomic ideal gas equation: The ideal gas law describes the behavior of an ideal gas, a hypothetical substance. Are Ideal Gases Diatomic.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free Are Ideal Gases Diatomic For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. Such a gas has more degrees of freedom than a monatomic gas. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic. Are Ideal Gases Diatomic.
From www.toppr.com
In a gas of diatomic molecules, the ratio of the two specific heats of Are Ideal Gases Diatomic The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^. Are Ideal Gases Diatomic.
From scoop.eduncle.com
15. for a diatomic ideal gas near room temperature. what fraction of Are Ideal Gases Diatomic \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that. Are Ideal Gases Diatomic.
From www.solutioninn.com
[Solved] A heat engine takes 0.350 mol of a diatom SolutionInn Are Ideal Gases Diatomic As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). The ideal gas law describes the behavior of. Are Ideal Gases Diatomic.
From www.solutionspile.com
[Solved] A diatomic ideal gas goes through the cycle a b Are Ideal Gases Diatomic For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). Such a gas has more degrees of freedom than a monatomic gas. \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. As per the kinetic theory of. Are Ideal Gases Diatomic.
From www.bartleby.com
Diatomic Gas bartleby Are Ideal Gases Diatomic Such a gas has more degrees of freedom than a monatomic gas. A diatomic ideal gas equation: \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles. Are Ideal Gases Diatomic.
From brainly.in
Explain monatomic diatomic and polyatomic gas molecule of specific heat Are Ideal Gases Diatomic \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy. Are Ideal Gases Diatomic.
From www.askiitians.com
An ideal diatomic gas is caused to pass through a cycle shown on the Are Ideal Gases Diatomic As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. Such a gas has more degrees of freedom than a monatomic gas. \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. For. Are Ideal Gases Diatomic.
From claire-yersbloghenderson.blogspot.com
The Smallest Complete Unit of a Compound or Diatomic Gas Are Ideal Gases Diatomic As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. A diatomic ideal gas equation: \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. The ideal gas law describes the behavior of. Are Ideal Gases Diatomic.
From askfilo.com
The temperature of 3.00 mol of an ideal diatomic gas is increased by 40.0.. Are Ideal Gases Diatomic \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. Such a gas has more degrees of freedom than a monatomic gas. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. As per the kinetic theory of ideal. Are Ideal Gases Diatomic.
From www.toppr.com
At a constant pressure, a given amount of ideal diatomic gas expands Are Ideal Gases Diatomic As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. Such a gas has more degrees of freedom than a monatomic gas. For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). A diatomic ideal gas equation: \ [ z (t, v, n)=\frac. Are Ideal Gases Diatomic.
From studylib.net
CHAPTER 6 IDEAL DIATOMIC GAS Are Ideal Gases Diatomic \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained. Are Ideal Gases Diatomic.
From sciencenotes.org
Real Gas vs Ideal Gas Are Ideal Gases Diatomic \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Such a. Are Ideal Gases Diatomic.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID4494868 Are Ideal Gases Diatomic For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. A diatomic ideal gas equation: Such a. Are Ideal Gases Diatomic.
From www.doubtnut.com
One mole of an ideal diatomic gas undergoes a transition from A to B a Are Ideal Gases Diatomic \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. A diatomic ideal gas equation: For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Such a. Are Ideal Gases Diatomic.
From www.toppr.com
An ideal diatomic gas is expanded so that the amount of heat Are Ideal Gases Diatomic As per the kinetic theory of ideal gases, ideal gases are considered to behave as point particles that occupy negligible volume,. \ [ z (t, v, n)=\frac {1} {n !}\left [\frac {v} {\lambda^ {3} (t)}\left (\frac {4 \pi^ {3} i k_ {b} t} {h^ {2}}\right)\right]^ {n}\] general. \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three. Are Ideal Gases Diatomic.
From askfilo.com
A diatomic ideal gas is used in a Carnot engine as the working substance... Are Ideal Gases Diatomic The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. A diatomic ideal gas equation: \(\delta{u} = \frac{5}{2}nr\delta{t}\) in a diatomic gas, it has a total of three translational kinetic. For example, consider a diatomic ideal gas (a good model for nitrogen, \(n_2\), and oxygen, \(o_2\)). Such a. Are Ideal Gases Diatomic.