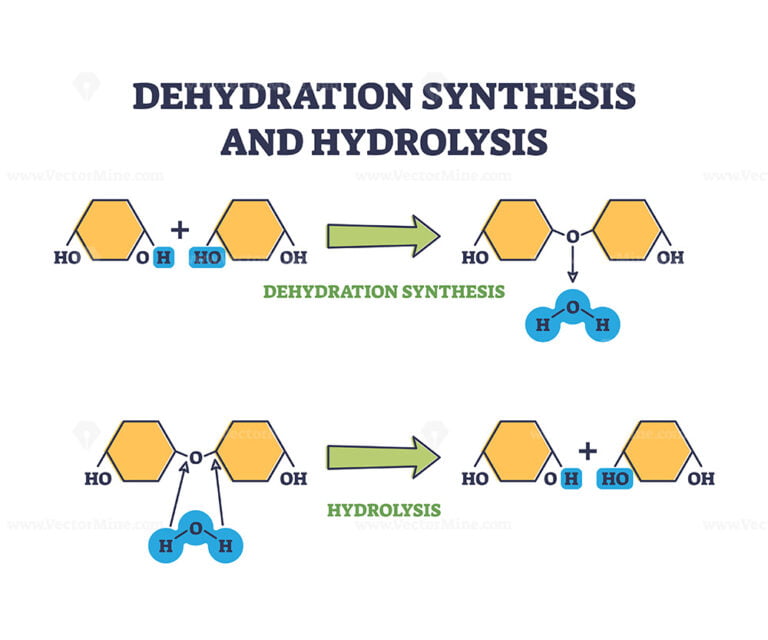
Synthesis Water Definition . The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. In doing so, monomers release water. In doing so, monomers release water. Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or water molecules. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. • water (h 2 o) is perhaps the most important chemical in all of biology and.
from vectormine.com
The monomers combine with each other via covalent bonds to form larger molecules known as polymers. In doing so, monomers release water. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. In doing so, monomers release water. • water (h 2 o) is perhaps the most important chemical in all of biology and. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or water molecules.
Dehydration synthesis and hydrolysis chemical process stages outline
Synthesis Water Definition In doing so, monomers release water. • water (h 2 o) is perhaps the most important chemical in all of biology and. In doing so, monomers release water. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or water molecules. In doing so, monomers release water. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. The monomers combine with each other via covalent bonds to form larger molecules known as polymers.
From royalsocietypublishing.org
Greener synthesis of chemical compounds and materials Royal Society Synthesis Water Definition Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. The monomers combine with each other via covalent bonds to form larger molecules. Synthesis Water Definition.
From www.pinterest.com
Chemical Reactions Types, Definitions, and Examples Teaching Synthesis Water Definition Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. In doing so, monomers release water. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. • water (h 2 o) is perhaps the most important chemical in all of biology. Synthesis Water Definition.
From www.pinterest.com
Difference Between Condensation and Hydrolysis Definition, Mechanism Synthesis Water Definition In doing so, monomers release water. In doing so, monomers release water. • water (h 2 o) is perhaps the most important chemical in all of biology and. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water. Synthesis Water Definition.
From www.youtube.com
How to Synthesize WATER SFX YouTube Synthesis Water Definition In doing so, monomers release water. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water. Synthesis Water Definition.
From schematicellsshyers.z21.web.core.windows.net
Protein Synthesis Diagram Step By Step Synthesis Water Definition In doing so, monomers release water. In doing so, monomers release water. Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or water molecules. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Define dehydration synthesis “dehydration reactions can. Synthesis Water Definition.
From www.tessshebaylo.com
Chemical Equation Synthesis Tessshebaylo Synthesis Water Definition Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing. Synthesis Water Definition.
From www.slideserve.com
PPT The 5 Types of Reactions PowerPoint Presentation, free download Synthesis Water Definition Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. In doing so, monomers release water. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. • water (h 2 o) is perhaps. Synthesis Water Definition.
From www.chemistrylearner.com
Dehydration Synthesis Definition, Examples, and Equations Synthesis Water Definition Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from. Synthesis Water Definition.
From en.wikipedia.org
Water cycle Wikipedia Synthesis Water Definition In doing so, monomers release water. In doing so, monomers release water. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. • water (h 2 o) is perhaps the most important chemical in all of biology and.. Synthesis Water Definition.
From www.biologyonline.com
Dehydration reaction Definition and Examples Biology Online Dictionary Synthesis Water Definition Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing. Synthesis Water Definition.
From present5.com
Chapter 8 Metabolism Enzymes AP Biology Synthesis Water Definition The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. In doing so, monomers release water. • water (h 2 o) is perhaps the most important chemical in all of. Synthesis Water Definition.
From philschatz.com
Compounds Essential to Human Functioning · Anatomy and Physiology Synthesis Water Definition The monomers combine with each other via covalent bonds to form larger molecules known as polymers. In doing so, monomers release water. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. The monomers combine with each other via covalent bonds to form larger molecules known. Synthesis Water Definition.
From kidspressmagazine.com
Photosynthesis Synthesis Water Definition In doing so, monomers release water. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. The monomers combine with each other via covalent bonds to form larger molecules known. Synthesis Water Definition.
From www.slideserve.com
PPT Synthesizing Texts PowerPoint Presentation, free download ID Synthesis Water Definition The monomers combine with each other via covalent bonds to form larger molecules known as polymers. In doing so, monomers release water. In doing so, monomers release water. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Dehydration synthesis is the reaction at which two small molecules react together to form a new. Synthesis Water Definition.
From www.britannica.com
Surfactant Definition, Properties, Examples, & Facts Britannica Synthesis Water Definition The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. In doing so, monomers release water. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming. Synthesis Water Definition.
From www.classroomnook.com
Reading Comprehension Strategy Series How to Teach Students to Synthesis Water Definition Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. In doing so, monomers release water. In doing so, monomers release water. The monomers combine with each other via covalent bonds. Synthesis Water Definition.
From www.studocu.com
Synthesis CHEMICAL REACTION A synthesis reaction or direct Synthesis Water Definition Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or water molecules. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. The monomers combine with each other via covalent bonds to form. Synthesis Water Definition.
From slideplayer.com
Biomolecule Processes ppt download Synthesis Water Definition In doing so, monomers release water. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. In doing so, monomers release water. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a. Synthesis Water Definition.
From www.masterorganicchemistry.com
Synthesis (4) Alkene Reaction Map, Including Alkyl Halide Reactions Synthesis Water Definition • water (h 2 o) is perhaps the most important chemical in all of biology and. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. In doing so, monomers release water. In doing so, monomers release water. The monomers combine with each other via covalent bonds to form larger molecules known as polymers.. Synthesis Water Definition.
From www.numerade.com
SOLVED '1. Define Photosynthesis the answer is The process by which Synthesis Water Definition In doing so, monomers release water. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming. Synthesis Water Definition.
From animalia-life.club
Dehydration Synthesis Synthesis Water Definition Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Dehydration synthesis, also known as. Synthesis Water Definition.
From www.britannica.com
Hydrolysis Definition, Examples, & Facts Britannica Synthesis Water Definition • water (h 2 o) is perhaps the most important chemical in all of biology and. In doing so, monomers release water. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Dehydration synthesis, also known as a. Synthesis Water Definition.
From www.researchgate.net
Synthesis of NaHSe. (a) Chemical formula. (b) Volume displacement Synthesis Water Definition The monomers combine with each other via covalent bonds to form larger molecules known as polymers. In doing so, monomers release water. In doing so, monomers release water. Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or water molecules. • water (h 2 o) is. Synthesis Water Definition.
From www.researchgate.net
Synthesis indexes and meaning. Download Scientific Diagram Synthesis Water Definition Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. In doing so, monomers release water. In doing so, monomers release water. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. • water (h 2 o) is perhaps the most. Synthesis Water Definition.
From www.worldatlas.com
Photosynthesis Explained WorldAtlas Synthesis Water Definition • water (h 2 o) is perhaps the most important chemical in all of biology and. In doing so, monomers release water. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. Define dehydration synthesis “dehydration reactions can. Synthesis Water Definition.
From vectormine.com
Dehydration synthesis and hydrolysis chemical process stages outline Synthesis Water Definition • dehydration synthesis is a reaction that combines molecules and results in a loss of water. In doing so, monomers release water. In doing so, monomers release water. Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or water molecules. The monomers combine with each other. Synthesis Water Definition.
From www.expii.com
Synthesis Reactions — Definition & Examples Expii Synthesis Water Definition In doing so, monomers release water. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. • water (h 2 o) is perhaps the most important chemical in all of biology and. In doing so, monomers release water.. Synthesis Water Definition.
From www.gbu-presnenskij.ru
Dehydration Synthesis Definition Examples Video Lesson, 42 OFF Synthesis Water Definition The monomers combine with each other via covalent bonds to form larger molecules known as polymers. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. • water (h 2 o) is perhaps the most important chemical in all of biology and. Define dehydration synthesis “dehydration. Synthesis Water Definition.
From www.researchgate.net
Schematic illustration of hydrothermal synthesis of FAp nanoparticles Synthesis Water Definition In doing so, monomers release water. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. • water (h 2 o) is perhaps the most important chemical in all of biology and. Dehydration synthesis is the reaction at which two small molecules react together to form. Synthesis Water Definition.
From study.com
Dehydration Synthesis Definition, Reaction & Examples Video & Lesson Synthesis Water Definition In doing so, monomers release water. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. • water (h 2 o) is perhaps the most important chemical in all of biology. Synthesis Water Definition.
From www.vrogue.co
Dehydration Synthesis Definition Reaction Examples Hy vrogue.co Synthesis Water Definition In doing so, monomers release water. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or water molecules. The monomers combine with each. Synthesis Water Definition.
From www.biologyonline.com
Biomolecule Definition and Examples Biology Online Dictionary Synthesis Water Definition Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or water molecules. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. The monomers combine with each other via covalent bonds to form. Synthesis Water Definition.
From sciencenotes.org
What Is a Synthesis Reaction? Definition and Examples Synthesis Water Definition • dehydration synthesis is a reaction that combines molecules and results in a loss of water. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. The monomers combine with each other via covalent bonds to form larger molecules known as polymers. • water (h 2. Synthesis Water Definition.
From www.pinterest.com
dehydration synthesis / hydrolysis Chemistry lessons, Chemistry Synthesis Water Definition • water (h 2 o) is perhaps the most important chemical in all of biology and. • dehydration synthesis is a reaction that combines molecules and results in a loss of water. Define dehydration synthesis “dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. Dehydration synthesis is the reaction. Synthesis Water Definition.
From www.researchgate.net
(a) Schematic diagram of the synthesis of MXene by HF etching [24]. (b Synthesis Water Definition In doing so, monomers release water. In doing so, monomers release water. Dehydration synthesis, also known as a dehydration reaction, is a chemical reaction that involves forming a new compound accompanied by removing water from the. Dehydration synthesis is the reaction at which two small molecules react together to form a new large molecule by removing a water molecule or. Synthesis Water Definition.