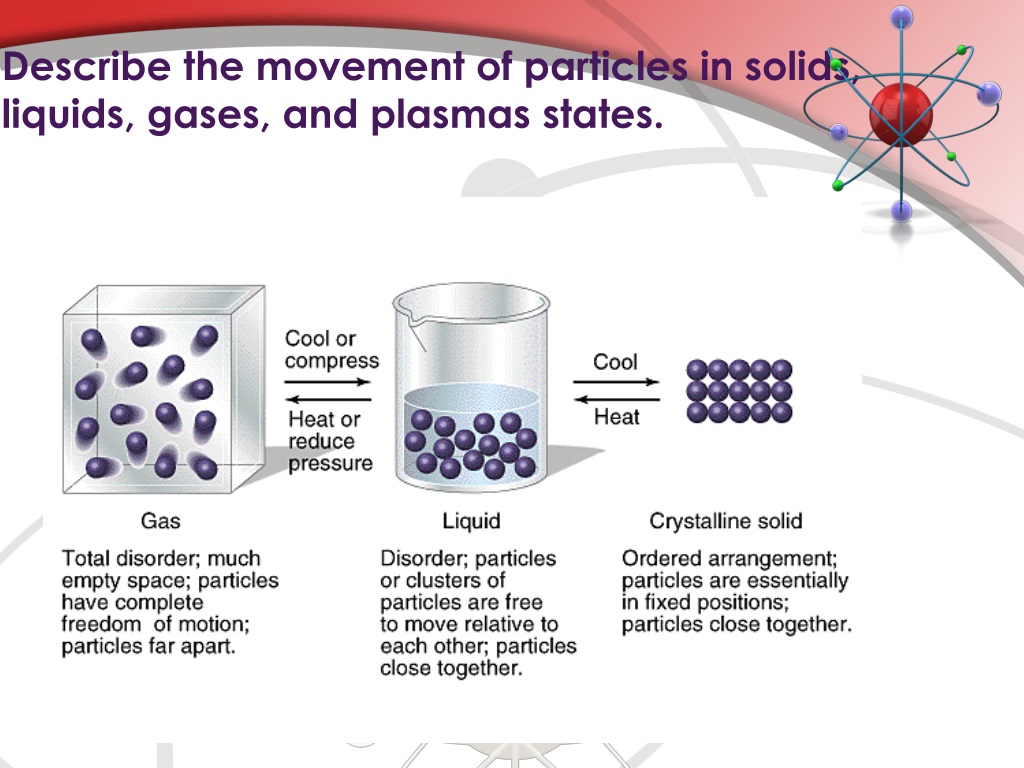
Solid Liquid Gas Particle Movement . The molecules of a liquid are packed relatively close together. Gas vibrate and move freely at high speeds. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. All substances are made from particles, and the forces. Solid vibrate (jiggle) but generally. Particle motion in a solid. This model explains the properties of. Particles move rapidly in all. In liquids, particles are quite close together and move with random motion throughout the container. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. How do particles move inside a solid, liquid and gas? Consequently, liquids are much denser than gases. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Liquid vibrate, move about, and slide past each other. In a solid, the particles can vibrate.
from www.slideserve.com
Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. The three states of matter can be represented by the particle model. The molecules of a liquid are packed relatively close together. Solid vibrate (jiggle) but generally. Consequently, liquids are much denser than gases. Particles move rapidly in all. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. How do particles move inside a solid, liquid and gas? This model explains the properties of. In a solid, the particles can vibrate.
PPT Matter PowerPoint Presentation, free download ID9590960
Solid Liquid Gas Particle Movement Particle motion in a solid. Liquid vibrate, move about, and slide past each other. The molecules of a liquid are packed relatively close together. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. The three states of matter can be represented by the particle model. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Gas vibrate and move freely at high speeds. Consequently, liquids are much denser than gases. In a solid, the particles can vibrate. In liquids, particles are quite close together and move with random motion throughout the container. How do particles move inside a solid, liquid and gas? Particles move rapidly in all. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. All substances are made from particles, and the forces. Particle motion in a solid. Solid vibrate (jiggle) but generally.
From stock.adobe.com
states of matter solids liquids and gases. Matter appears in three Solid Liquid Gas Particle Movement The three states of matter can be represented by the particle model. In liquids, particles are quite close together and move with random motion throughout the container. In a solid, the particles can vibrate. This model explains the properties of. The molecules of a liquid are packed relatively close together. Particle motion in a solid. Liquid vibrate, move about, and. Solid Liquid Gas Particle Movement.
From aaliyah-bogspotcervantes.blogspot.com
Describe the Movement of Particles in Solids Liquids and Gases Solid Liquid Gas Particle Movement In a solid, the particles can vibrate. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. All substances are made from particles, and the forces. Liquid vibrate, move about, and slide past each other. How do particles move inside a solid, liquid and gas? In a. Solid Liquid Gas Particle Movement.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Solid Liquid Gas Particle Movement In liquids, particles are quite close together and move with random motion throughout the container. Consequently, liquids are much denser than gases. Particles move rapidly in all. In a solid, the particles can vibrate. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The molecules of a liquid are packed relatively. Solid Liquid Gas Particle Movement.
From www.alamy.com
Gas solid and liquid hires stock photography and images Alamy Solid Liquid Gas Particle Movement How do particles move inside a solid, liquid and gas? Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. In a solid, the particles can vibrate. This model explains the properties of. Particle motion in a solid. The molecules of a liquid are packed relatively close together. Particles move rapidly in. Solid Liquid Gas Particle Movement.
From owlcation.com
What Is the Particle Model? A Guide to Solids, Liquids and Gases Solid Liquid Gas Particle Movement Particle motion in a solid. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. Liquid vibrate, move about, and slide past each other. In liquids, particles are quite close together and move with random motion throughout the container. Particles move rapidly in all. Gas vibrate and. Solid Liquid Gas Particle Movement.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid Liquid Gas Particle Movement The three states of matter can be represented by the particle model. In a solid, the particles can vibrate. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. The molecules of a liquid are packed relatively close together. All substances are made from particles, and the forces. Particle motion in. Solid Liquid Gas Particle Movement.
From chasityathuff.blogspot.com
Movement of Particles in a Solid ChasityatHuff Solid Liquid Gas Particle Movement In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. This model explains the properties of. In liquids, particles are quite close together and move with random motion throughout the container. Particles move rapidly in all. Solids and liquids have particles that are fairly close to one another, and are thus. Solid Liquid Gas Particle Movement.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid Liquid Gas Particle Movement The molecules of a liquid are packed relatively close together. Consequently, liquids are much denser than gases. In liquids, particles are quite close together and move with random motion throughout the container. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Heat, cool and compress atoms and molecules and watch. Solid Liquid Gas Particle Movement.
From www.oxnotes.com
States of Matter Revision Notes IGCSE Chemistry OxNotes GCSE Revision Solid Liquid Gas Particle Movement In a solid, the particles can vibrate. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Particles move rapidly in all. Solid vibrate (jiggle) but generally. Consequently, liquids are much denser than. Solid Liquid Gas Particle Movement.
From educationspares.z4.web.core.windows.net
What Is Particle Motion Solid Liquid Gas Particle Movement The three states of matter can be represented by the particle model. Gas vibrate and move freely at high speeds. Particle motion in a solid. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. All substances are made from particles, and the forces. This model explains the properties of. The molecules. Solid Liquid Gas Particle Movement.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid Liquid Gas Particle Movement Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Consequently, liquids are much denser than gases. The three states of matter can be represented by the particle model. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Solids and liquids have. Solid Liquid Gas Particle Movement.
From stock.adobe.com
states of matter solids liquids and gases. Vector illustration isolated Solid Liquid Gas Particle Movement In liquids, particles are quite close together and move with random motion throughout the container. All substances are made from particles, and the forces. How do particles move inside a solid, liquid and gas? This model explains the properties of. Gas vibrate and move freely at high speeds. Solid vibrate (jiggle) but generally. Particle motion in a solid. In a. Solid Liquid Gas Particle Movement.
From msreinders.weebly.com
States of Matter Shaw Middle School Science Solid Liquid Gas Particle Movement Particle motion in a solid. This model explains the properties of. Liquid vibrate, move about, and slide past each other. In a solid, the particles can vibrate. Gas vibrate and move freely at high speeds. In liquids, particles are quite close together and move with random motion throughout the container. The three states of matter can be represented by the. Solid Liquid Gas Particle Movement.
From www.snexplores.org
Explainer What are the different states of matter? Solid Liquid Gas Particle Movement Particle motion in a solid. How do particles move inside a solid, liquid and gas? Particles move rapidly in all. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas. Solid Liquid Gas Particle Movement.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid Liquid Gas Particle Movement In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Liquid vibrate, move about, and slide past each other. The three states of matter can be represented by the particle model. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish. Solid Liquid Gas Particle Movement.
From www.explainthatstuff.com
States of matter A simple introduction to solids, liquids, gases Solid Liquid Gas Particle Movement The molecules of a liquid are packed relatively close together. Liquid vibrate, move about, and slide past each other. The three states of matter can be represented by the particle model. Gas vibrate and move freely at high speeds. All substances are made from particles, and the forces. Heat, cool and compress atoms and molecules and watch as they change. Solid Liquid Gas Particle Movement.
From usermanualwhelming.z21.web.core.windows.net
Solid Liquid Gas Particle Diagram Solid Liquid Gas Particle Movement Consequently, liquids are much denser than gases. This model explains the properties of. Particles move rapidly in all. Gas vibrate and move freely at high speeds. Particle motion in a solid. The molecules of a liquid are packed relatively close together. Liquid vibrate, move about, and slide past each other. Heat, cool and compress atoms and molecules and watch as. Solid Liquid Gas Particle Movement.
From www.elevise.co.uk
C2 I) States of Matter AQA Combined Science Trilogy Elevise Solid Liquid Gas Particle Movement Consequently, liquids are much denser than gases. The three states of matter can be represented by the particle model. This model explains the properties of. Solid vibrate (jiggle) but generally. In a solid, the particles can vibrate. How do particles move inside a solid, liquid and gas? All substances are made from particles, and the forces. In a solid like. Solid Liquid Gas Particle Movement.
From www.dreamstime.com
States of Matter. Vector Circles Infographic Illustration Stock Vector Solid Liquid Gas Particle Movement Consequently, liquids are much denser than gases. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The three states of matter can be represented by the particle model. Gas vibrate and move freely at high speeds. The molecules of a liquid are packed relatively close together. Liquid vibrate, move about, and. Solid Liquid Gas Particle Movement.
From www.slideserve.com
PPT Matter PowerPoint Presentation, free download ID9590960 Solid Liquid Gas Particle Movement In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Solid vibrate (jiggle) but generally. This model explains the properties of. The molecules of a liquid are packed relatively close together. In a solid, the particles can vibrate. How do particles move inside a solid, liquid and gas? Liquid vibrate, move. Solid Liquid Gas Particle Movement.
From www.expii.com
What Are the Phases of Matter? — Overview & Examples Expii Solid Liquid Gas Particle Movement Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. How do particles move inside a solid, liquid and gas? Solid vibrate (jiggle) but generally. Particle motion in a solid. Particles move rapidly in all. Consequently, liquids are much denser than gases. In a solid like this brick, the particles are regularly. Solid Liquid Gas Particle Movement.
From www.bbc.co.uk
What is the arrangement of particles in a solid, liquid and gas? BBC Solid Liquid Gas Particle Movement This model explains the properties of. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. The molecules of a liquid are packed relatively close together. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. In a solid,. Solid Liquid Gas Particle Movement.
From thehungryjpeg.com
Different states of matter solid, liquid, gas vector diagram By Solid Liquid Gas Particle Movement Solid vibrate (jiggle) but generally. The molecules of a liquid are packed relatively close together. The three states of matter can be represented by the particle model. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Particle motion in a solid. In a solid like this brick, the particles are regularly. Solid Liquid Gas Particle Movement.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid Liquid Gas Particle Movement This model explains the properties of. How do particles move inside a solid, liquid and gas? Solid vibrate (jiggle) but generally. Particle motion in a solid. In liquids, particles are quite close together and move with random motion throughout the container. Liquid vibrate, move about, and slide past each other. The three states of matter can be represented by the. Solid Liquid Gas Particle Movement.
From chemstuff.co.uk
Particle Model of Solids, Liquids and Gases Chemstuff Solid Liquid Gas Particle Movement In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. In liquids, particles are quite close together and move with random motion throughout the container. Gas vibrate and move freely at high speeds. Particle motion in a solid. All substances are made from particles, and the forces. Solid vibrate (jiggle) but. Solid Liquid Gas Particle Movement.
From www.youtube.com
Motion of particles in solid, liquid and gases (animated video Solid Liquid Gas Particle Movement All substances are made from particles, and the forces. Particles move rapidly in all. Gas vibrate and move freely at high speeds. The molecules of a liquid are packed relatively close together. Particle motion in a solid. In liquids, particles are quite close together and move with random motion throughout the container. Heat, cool and compress atoms and molecules and. Solid Liquid Gas Particle Movement.
From www.numerade.com
SOLVEDExamine Figure 1.12 . In which physical state do particles have Solid Liquid Gas Particle Movement Liquid vibrate, move about, and slide past each other. The three states of matter can be represented by the particle model. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. In a solid, the particles can vibrate. All substances are made from particles, and the forces.. Solid Liquid Gas Particle Movement.
From www.pngkey.com
Download Particle Arrangements Arrangement Of Particles In Solid Solid Liquid Gas Particle Movement Particle motion in a solid. The three states of matter can be represented by the particle model. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. In liquids, particles are quite close. Solid Liquid Gas Particle Movement.
From www.youtube.com
The arrangement of particles in solids, liquids and gases Edukite Solid Liquid Gas Particle Movement The molecules of a liquid are packed relatively close together. Gas vibrate and move freely at high speeds. Particle motion in a solid. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. The three states of matter can be represented by the particle model. In liquids, particles are quite close. Solid Liquid Gas Particle Movement.
From rohanexmoses.blogspot.com
Solid to Gas is Called RohanexMoses Solid Liquid Gas Particle Movement Particles move rapidly in all. In liquids, particles are quite close together and move with random motion throughout the container. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The three states of matter can be represented by the particle model. Solid vibrate (jiggle) but generally. Gas vibrate and move freely. Solid Liquid Gas Particle Movement.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Solid Liquid Gas Particle Movement Particle motion in a solid. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. All substances are made from particles, and the forces. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Gas vibrate and move. Solid Liquid Gas Particle Movement.
From royce-bogspotmccarty.blogspot.com
Describe the Movement of the Particles in the Air Solid Liquid Gas Particle Movement Gas vibrate and move freely at high speeds. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. All substances are made from particles, and the forces. Solid vibrate (jiggle) but generally. Liquid vibrate, move about, and slide past each other. Particles move rapidly in all. This model explains the properties. Solid Liquid Gas Particle Movement.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid Liquid Gas Particle Movement In a solid, the particles can vibrate. The molecules of a liquid are packed relatively close together. Particles move rapidly in all. Liquid vibrate, move about, and slide past each other. Solids and liquids have particles that are fairly close to one another, and are thus called condensed phases to distinguish them from gases. Consequently, liquids are much denser than. Solid Liquid Gas Particle Movement.
From byjus.com
Draw a sketch to show the arrangement of particles in solid, liquid and Solid Liquid Gas Particle Movement In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. In liquids, particles are quite close together and move with random motion throughout the container. How do particles move inside a solid, liquid and gas? This model explains the properties of. Particle motion in a solid. Gas vibrate and move freely. Solid Liquid Gas Particle Movement.
From johnwest.edublogs.org
Solids liquids and gasses, a story in three parts. Science Connected Solid Liquid Gas Particle Movement Consequently, liquids are much denser than gases. In liquids, particles are quite close together and move with random motion throughout the container. The three states of matter can be represented by the particle model. This model explains the properties of. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. All substances. Solid Liquid Gas Particle Movement.