Zinc And Copper Oxide Reaction . redox reactions are only spontaneous in one direction. in the first reaction, the copper ion is able to oxidize the zinc metal. By observing this reaction and its products,. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. demonstrating redox reaction by reducing copper oxide to. I) oxide and zinc metal are reacted together. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. the reaction between zinc and 42 copper oxide. However, in the second reaction, the zinc ion is not able to. reaction between zinc and copper (ii) oxide.
from blog.thepipingmart.com
in the first reaction, the copper ion is able to oxidize the zinc metal. By observing this reaction and its products,. I) oxide and zinc metal are reacted together. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. reaction between zinc and copper (ii) oxide. However, in the second reaction, the zinc ion is not able to. redox reactions are only spontaneous in one direction. demonstrating redox reaction by reducing copper oxide to. the reaction between zinc and 42 copper oxide. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper.
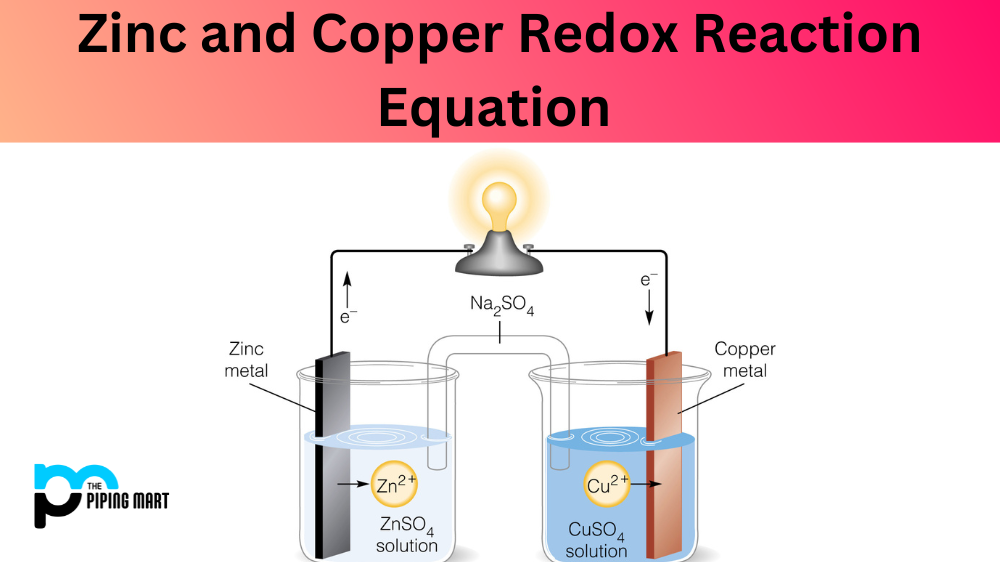
Zinc and Copper Redox Reaction Equation
Zinc And Copper Oxide Reaction redox reactions are only spontaneous in one direction. the reaction between zinc and 42 copper oxide. in the first reaction, the copper ion is able to oxidize the zinc metal. I) oxide and zinc metal are reacted together. By observing this reaction and its products,. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. redox reactions are only spontaneous in one direction. demonstrating redox reaction by reducing copper oxide to. reaction between zinc and copper (ii) oxide. However, in the second reaction, the zinc ion is not able to. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of.
From www.numerade.com
SOLVED Which methods are suitable for preparing both zinc sulfate and Zinc And Copper Oxide Reaction demonstrating redox reaction by reducing copper oxide to. However, in the second reaction, the zinc ion is not able to. redox reactions are only spontaneous in one direction. the reaction between zinc and 42 copper oxide. reaction between zinc and copper (ii) oxide. By observing this reaction and its products,. cu + zno = cuo. Zinc And Copper Oxide Reaction.
From en.ppt-online.org
Metals online presentation Zinc And Copper Oxide Reaction in the first reaction, the copper ion is able to oxidize the zinc metal. the reaction between zinc and 42 copper oxide. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. reaction between zinc and copper (ii) oxide. demonstrating redox reaction by reducing copper oxide to. I). Zinc And Copper Oxide Reaction.
From www.youtube.com
Reaction between zinc and copper(II) oxide YouTube Zinc And Copper Oxide Reaction cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. However, in the second reaction, the zinc ion is not able to. redox reactions are only spontaneous in one direction. I) oxide and zinc metal are reacted together. By observing this reaction and its products,.. Zinc And Copper Oxide Reaction.
From slideplayer.com
9F Patterns of reactivity ppt download Zinc And Copper Oxide Reaction cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. However, in the second reaction, the zinc ion is not able to. reaction between zinc and copper (ii) oxide. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide. Zinc And Copper Oxide Reaction.
From classnotes.org.in
Extraction of Copper and Zinc Chemistry, Class 12, General Principles Zinc And Copper Oxide Reaction reaction between zinc and copper (ii) oxide. redox reactions are only spontaneous in one direction. However, in the second reaction, the zinc ion is not able to. I) oxide and zinc metal are reacted together. in the first reaction, the copper ion is able to oxidize the zinc metal. cu + zno = cuo + zn. Zinc And Copper Oxide Reaction.
From www.teachoo.com
Chemical Properties of Metals [with Reaction Examples] Teachoo Zinc And Copper Oxide Reaction I) oxide and zinc metal are reacted together. the reaction between zinc and 42 copper oxide. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. redox reactions are only spontaneous in one direction. in the first reaction, the copper ion is able. Zinc And Copper Oxide Reaction.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Oxide Reaction cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. However, in the second reaction, the zinc ion is not able to. in the first reaction, the copper ion is able to oxidize the zinc metal. reaction between zinc and copper (ii) oxide. . Zinc And Copper Oxide Reaction.
From classnotes.org.in
Extraction of Copper and Zinc Chemistry, Class 12, General Principles Zinc And Copper Oxide Reaction However, in the second reaction, the zinc ion is not able to. I) oxide and zinc metal are reacted together. the reaction between zinc and 42 copper oxide. redox reactions are only spontaneous in one direction. demonstrating redox reaction by reducing copper oxide to. cu + zno = cuo + zn is a single displacement (substitution). Zinc And Copper Oxide Reaction.
From www.studypool.com
SOLUTION Cfns experiment 42 the reaction between zinc and copper oxide Zinc And Copper Oxide Reaction By observing this reaction and its products,. However, in the second reaction, the zinc ion is not able to. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. I) oxide and zinc metal are reacted together. redox reactions are only spontaneous in one direction.. Zinc And Copper Oxide Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs Zinc And Copper Oxide Reaction I) oxide and zinc metal are reacted together. However, in the second reaction, the zinc ion is not able to. By observing this reaction and its products,. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. in the first reaction, the copper ion is able to oxidize the zinc metal.. Zinc And Copper Oxide Reaction.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Zinc And Copper Oxide Reaction reaction between zinc and copper (ii) oxide. I) oxide and zinc metal are reacted together. the reaction between zinc and 42 copper oxide. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. in the first reaction, the copper ion is able to. Zinc And Copper Oxide Reaction.
From bioengineer.org
Zinc oxide key component for the methanol synthesis reaction over copper Zinc And Copper Oxide Reaction redox reactions are only spontaneous in one direction. the reaction between zinc and 42 copper oxide. By observing this reaction and its products,. However, in the second reaction, the zinc ion is not able to. reaction between zinc and copper (ii) oxide. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc. Zinc And Copper Oxide Reaction.
From docslib.org
42 the Reaction Between Zinc and Copper Oxide DocsLib Zinc And Copper Oxide Reaction I) oxide and zinc metal are reacted together. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. the reaction between zinc and 42 copper oxide. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. . Zinc And Copper Oxide Reaction.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Zinc And Copper Oxide Reaction in the first reaction, the copper ion is able to oxidize the zinc metal. By observing this reaction and its products,. However, in the second reaction, the zinc ion is not able to. I) oxide and zinc metal are reacted together. demonstrating redox reaction by reducing copper oxide to. redox reactions are only spontaneous in one direction.. Zinc And Copper Oxide Reaction.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc And Copper Oxide Reaction cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. I) oxide and zinc metal are reacted together. redox reactions are only spontaneous in one direction. However, in the second reaction, the zinc ion is not able to. demonstrating redox reaction by reducing copper. Zinc And Copper Oxide Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs Zinc And Copper Oxide Reaction cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. redox reactions are only spontaneous in one direction. However, in the second reaction, the zinc ion is not able to. reaction between zinc and copper (ii) oxide. the reaction between zinc and 42. Zinc And Copper Oxide Reaction.
From www.google.com
Patent US8623220 Synthesis of copper oxidedoped zincoxide Zinc And Copper Oxide Reaction I) oxide and zinc metal are reacted together. redox reactions are only spontaneous in one direction. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. However, in the second reaction, the zinc ion is not able to. in the first reaction, the copper ion is able to oxidize the. Zinc And Copper Oxide Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction copper zinc Fundamental Photographs Zinc And Copper Oxide Reaction in the first reaction, the copper ion is able to oxidize the zinc metal. reaction between zinc and copper (ii) oxide. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. demonstrating redox reaction by reducing copper oxide to. I) oxide and zinc metal are reacted together. By observing. Zinc And Copper Oxide Reaction.
From blog.thepipingmart.com
Metallic Oxides of Zinc, Magnesium, and Copper Zinc And Copper Oxide Reaction in the first reaction, the copper ion is able to oxidize the zinc metal. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. reaction between zinc and copper (ii) oxide. demonstrating redox reaction by reducing copper oxide to. the reaction between. Zinc And Copper Oxide Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs Zinc And Copper Oxide Reaction in the first reaction, the copper ion is able to oxidize the zinc metal. redox reactions are only spontaneous in one direction. I) oxide and zinc metal are reacted together. demonstrating redox reaction by reducing copper oxide to. the reaction between zinc and 42 copper oxide. cu + zno = cuo + zn is a. Zinc And Copper Oxide Reaction.
From blog.thepipingmart.com
Comparing the Reactivity of Copper vs Zinc Zinc And Copper Oxide Reaction By observing this reaction and its products,. However, in the second reaction, the zinc ion is not able to. the reaction between zinc and 42 copper oxide. redox reactions are only spontaneous in one direction. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. reaction between zinc and. Zinc And Copper Oxide Reaction.
From www.coursehero.com
[Solved] link for the experiment https//youtu.be/ILJhI43wVA Part Zinc And Copper Oxide Reaction reaction between zinc and copper (ii) oxide. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. in the first reaction, the copper ion is able to oxidize the zinc metal. the reaction between zinc and 42 copper oxide. I) oxide and zinc. Zinc And Copper Oxide Reaction.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Zinc And Copper Oxide Reaction cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. the reaction between zinc and 42 copper oxide. reaction between zinc and copper (ii) oxide. By observing this reaction and its products,. demonstrating redox reaction by reducing copper oxide to. However, in the. Zinc And Copper Oxide Reaction.
From exoinwrqp.blob.core.windows.net
Zinc Oxide Formula Charge at Bruce Clark blog Zinc And Copper Oxide Reaction reaction between zinc and copper (ii) oxide. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. in the first reaction, the copper ion is able to oxidize the zinc metal. However, in the second reaction, the zinc ion is not able to. the reaction between zinc and 42. Zinc And Copper Oxide Reaction.
From www.youtube.com
Copper 2+ ion reacting with solid zinc YouTube Zinc And Copper Oxide Reaction in the first reaction, the copper ion is able to oxidize the zinc metal. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. reaction between zinc and copper (ii) oxide. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper. Zinc And Copper Oxide Reaction.
From www.numerade.com
SOLVED Zinc metal can be obtained from zinc oxide (ZnO) by reacting Zinc And Copper Oxide Reaction in the first reaction, the copper ion is able to oxidize the zinc metal. However, in the second reaction, the zinc ion is not able to. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. reaction between zinc and copper (ii) oxide. redox reactions are only spontaneous in. Zinc And Copper Oxide Reaction.
From dxohphgdt.blob.core.windows.net
Copper Oxide Equation at Dora Longstreet blog Zinc And Copper Oxide Reaction copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. I) oxide and zinc metal are reacted together. demonstrating redox reaction by reducing copper oxide to. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. . Zinc And Copper Oxide Reaction.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc And Copper Oxide Reaction By observing this reaction and its products,. However, in the second reaction, the zinc ion is not able to. reaction between zinc and copper (ii) oxide. demonstrating redox reaction by reducing copper oxide to. redox reactions are only spontaneous in one direction. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc. Zinc And Copper Oxide Reaction.
From socratic.org
Can you describe the process that releases electrons in a zinc copper Zinc And Copper Oxide Reaction cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. the reaction between zinc and 42 copper oxide. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. However, in the second reaction, the zinc ion is. Zinc And Copper Oxide Reaction.
From www.sciencephoto.com
Copper oxide and zinc reaction Stock Image A500/0682 Science Zinc And Copper Oxide Reaction demonstrating redox reaction by reducing copper oxide to. redox reactions are only spontaneous in one direction. By observing this reaction and its products,. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. the reaction between zinc and 42 copper oxide. I) oxide and zinc metal are reacted together.. Zinc And Copper Oxide Reaction.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Zinc And Copper Oxide Reaction redox reactions are only spontaneous in one direction. I) oxide and zinc metal are reacted together. demonstrating redox reaction by reducing copper oxide to. By observing this reaction and its products,. However, in the second reaction, the zinc ion is not able to. the reaction between zinc and 42 copper oxide. copper(ii) oxide and zinc metal. Zinc And Copper Oxide Reaction.
From byjus.com
14.During indirect redox reaction why there is a release of zinc ions Zinc And Copper Oxide Reaction cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. By observing this reaction and its products,. However, in the second reaction, the zinc ion is not able to. reaction between zinc and copper (ii) oxide. the reaction between zinc and 42 copper oxide.. Zinc And Copper Oxide Reaction.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc And Copper Oxide Reaction copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. reaction between zinc and copper (ii) oxide. demonstrating redox reaction by reducing copper oxide to. redox reactions are only spontaneous in one direction. By observing this reaction and its products,. the reaction between zinc and 42 copper oxide.. Zinc And Copper Oxide Reaction.
From edu.rsc.org
Reacting zinc and copper(II) oxide Experiment RSC Education Zinc And Copper Oxide Reaction By observing this reaction and its products,. cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of. the reaction between zinc and 42 copper oxide. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. in. Zinc And Copper Oxide Reaction.
From www.slideserve.com
PPT Electrochemistry I PowerPoint Presentation, free download ID Zinc And Copper Oxide Reaction However, in the second reaction, the zinc ion is not able to. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. the reaction between zinc and 42 copper oxide. redox reactions are only spontaneous in one direction. cu + zno = cuo + zn is a single displacement. Zinc And Copper Oxide Reaction.