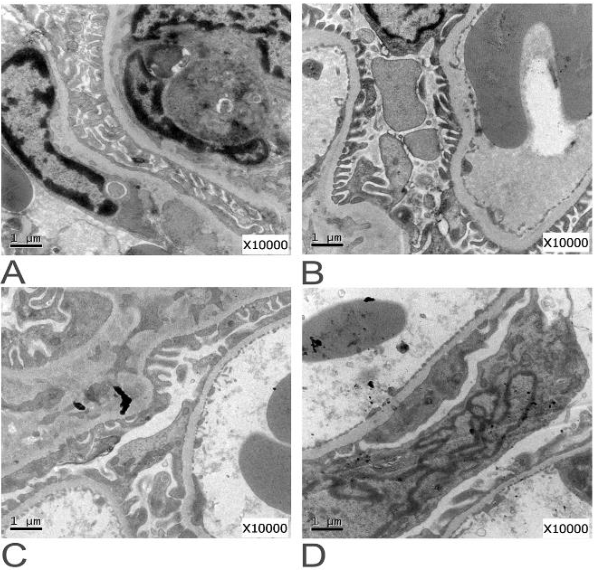
Proteinuria Sirolimus . The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006).
from www.semanticscholar.org
Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. This increased to 22.9% postswitch (p = 0.006).
Figure 1 from The role of sirolimus in proteinuria in diabetic
Proteinuria Sirolimus Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2.
From www.semanticscholar.org
Figure 1 from Increase of proteinuria after conversion from calcineurin Proteinuria Sirolimus Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria Sirolimus.
From www.researchgate.net
The mode of action of sirolimus sirolimus binds to the FKbinding Proteinuria Sirolimus Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. This increased to 22.9% postswitch (p = 0.006). Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria Sirolimus.
From www.researchgate.net
Diffusion of sirolimus into smooth muscle cells. © Cordis Corporation Proteinuria Sirolimus The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). Proteinuria Sirolimus.
From everyone.org
Buy Fyarro (sirolimus proteinbound particles (albuminbound)) Online Proteinuria Sirolimus Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Proteinuria Sirolimus.
From www.slideserve.com
PPT Renal Transplantation PowerPoint Presentation ID201710 Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria Sirolimus.
From www.slideserve.com
PPT Renal Transplantation PowerPoint Presentation ID201710 Proteinuria Sirolimus Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). Proteinuria Sirolimus.
From www.vinmec.com
Uses of Sirolimus ProteinBound Vinmec Proteinuria Sirolimus The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. This increased to 22.9% postswitch (p = 0.006). Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria Sirolimus.
From www.researchgate.net
Sirolimus treatment for two and seven days does not alter protein Proteinuria Sirolimus Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). Proteinuria Sirolimus.
From favpng.com
FKBP5 Tacrolimus Protein Sirolimus, PNG, 923x689px, Fkbp, Area, Biology Proteinuria Sirolimus Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria Sirolimus.
From slidetodoc.com
Proteinuria with sirolimus therapy Christophe Legendre Hpital Necker Proteinuria Sirolimus The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria Sirolimus.
From www.wikiwand.com
Sirolimus Wikiwand Proteinuria Sirolimus The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. This increased to 22.9% postswitch (p = 0.006). Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Proteinuria Sirolimus.
From www.researchgate.net
Efficacy of sirolimus in active SLE patients. Mean SLEDAI2K score (A Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria Sirolimus.
From www.researchgate.net
(PDF) The role of sirolimus in proteinuria in diabetic nephropathy rats Proteinuria Sirolimus Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria Sirolimus.
From www.researchgate.net
(PDF) Sirolimus reduces vasculopathy but exacerbates proteinuria in Proteinuria Sirolimus The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria Sirolimus.
From www.ajkd.org
Sirolimus Therapy of Focal Segmental Glomerulosclerosis Is Associated Proteinuria Sirolimus Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. This increased to 22.9% postswitch (p = 0.006). Proteinuria Sirolimus.
From slideplayer.com
Why Do Patients Develop Proteinuria With Sirolimus ppt download Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria Sirolimus.
From www.academia.edu
(PDF) Sirolimus is not always responsible for newonset proteinuria Proteinuria Sirolimus Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria Sirolimus.
From www.semanticscholar.org
Table 1 from Influence of sirolimus on proteinuria in de novo kidney Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria Sirolimus.
From www.semanticscholar.org
Figure 1 from The role of sirolimus in proteinuria in diabetic Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria Sirolimus.
From www.ajkd.org
Sirolimus Therapy of Focal Segmental Glomerulosclerosis Is Associated Proteinuria Sirolimus Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. This increased to 22.9% postswitch (p = 0.006). Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Proteinuria Sirolimus.
From www.researchgate.net
effect of sirolimus (s) on the renal expression of phosphorylated s6 Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Proteinuria Sirolimus.
From www.semanticscholar.org
Figure 1 from Sirolimus and proteinuria in kidney transplantation Proteinuria Sirolimus Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria Sirolimus.
From www.researchgate.net
FKBP12 and FKBP51 protein expressions in Tacrolimus, Sirolimusand Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria Sirolimus.
From www.ajkd.org
Why Do Patients Develop Proteinuria With Sirolimus? Do We Have the Proteinuria Sirolimus Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. This increased to 22.9% postswitch (p = 0.006). Proteinuria Sirolimus.
From www.researchgate.net
Efficacy of sirolimus on cSLE 24hr urine protein at each time point Proteinuria Sirolimus Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. This increased to 22.9% postswitch (p = 0.006). Proteinuria Sirolimus.
From www.researchgate.net
(PDF) A292 IMPACT OF SIROLIMUS PROTEINURIA FOLLOWING LIVER TRANSPLANTATION Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria Sirolimus.
From www.researchgate.net
Effect of late initiation of sirolimus on the progression renal Proteinuria Sirolimus The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. This increased to 22.9% postswitch (p = 0.006). Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Proteinuria Sirolimus.
From www.researchgate.net
Longitudinal changes in (A) proteinuria (B) estimated GFR (C) serum C3 Proteinuria Sirolimus Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria Sirolimus.
From www.academia.edu
(PDF) Influence of sirolimus on proteinuria in de novo kidney Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria Sirolimus.
From www.ahajournals.org
SirolimusFKBP12.6 Impairs Endothelial Barrier Function Through Protein Proteinuria Sirolimus Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria Sirolimus.
From www.researchgate.net
WT1 immunohistochemistry. Panel A C renal biopsies from Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Proteinuria Sirolimus.
From www.researchgate.net
(PDF) Proteinuria following conversion from azathioprine to sirolimus Proteinuria Sirolimus The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. This increased to 22.9% postswitch (p = 0.006). Proteinuria Sirolimus.
From www.fyarrohcp.com
FYARRO® (sirolimus proteinbound particles for injectable suspension Proteinuria Sirolimus Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Proteinuria Sirolimus.
From www.researchgate.net
The mode of action of sirolimus sirolimus binds to the FKbinding Proteinuria Sirolimus This increased to 22.9% postswitch (p = 0.006). The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. Proteinuria Sirolimus.
From biopharmanotes.com
Sirolimus BioPharma Notes Proteinuria Sirolimus Sirolimus was initially developed as a potential solution to the problem of chronic calcineurin inhibitor (cni) nephrotoxicity.1,2. Proteinuria is an increasingly recognized effect of sirolimus (srl) therapy in kidney transplant recipients. The heavy proteinuria developed after initiation of sirolimus‐tacrolimus immunosuppressive therapy and resolved after sirolimus withdrawal,. This increased to 22.9% postswitch (p = 0.006). Proteinuria Sirolimus.