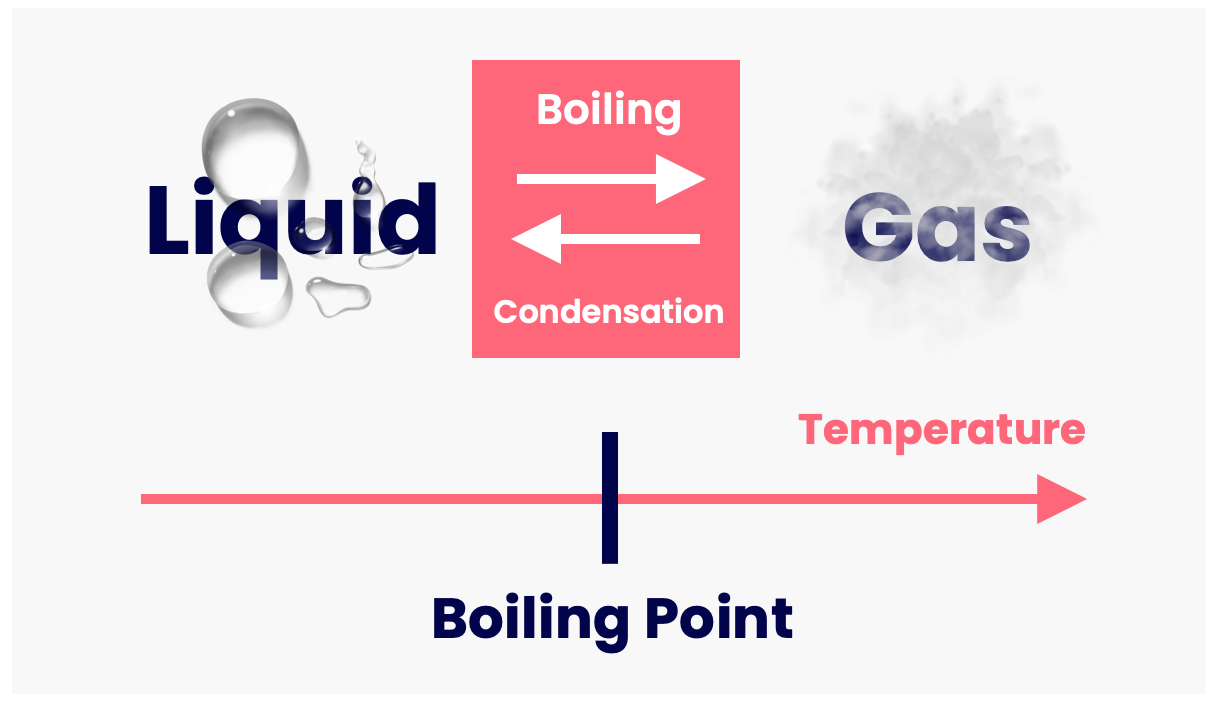
Characteristics Of Boiling Point For Mixture . the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. the characteristics of the substances determine the boiling and the melting point of the mixture. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. we'll start with the boiling points of pure a and b. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; Since b has the higher vapor pressure, it will have the lower boiling. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the.
from www.physicsfox.org
Since b has the higher vapor pressure, it will have the lower boiling. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; the characteristics of the substances determine the boiling and the melting point of the mixture. the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. we'll start with the boiling points of pure a and b. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the.
Melting & Boiling • Matter • Physics Fox
Characteristics Of Boiling Point For Mixture Since b has the higher vapor pressure, it will have the lower boiling. Since b has the higher vapor pressure, it will have the lower boiling. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. the characteristics of the substances determine the boiling and the melting point of the mixture. we'll start with the boiling points of pure a and b. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and Characteristics Of Boiling Point For Mixture the characteristics of the substances determine the boiling and the melting point of the mixture. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. the boiling point is defined as the temperature at which. Characteristics Of Boiling Point For Mixture.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Characteristics Of Boiling Point For Mixture the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; Since b has the higher vapor pressure, it will have the lower boiling.. Characteristics Of Boiling Point For Mixture.
From www.worksheetsplanet.com
What is Boiling Point Characteristics Of Boiling Point For Mixture the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. the characteristics of the substances determine the boiling and the melting point of the mixture. the boiling point is defined as the temperature at which. Characteristics Of Boiling Point For Mixture.
From www.slideserve.com
PPT Opening Assignment PowerPoint Presentation, free download ID Characteristics Of Boiling Point For Mixture Since b has the higher vapor pressure, it will have the lower boiling. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. the boiling point is defined as the temperature at which a liquid’s saturated. Characteristics Of Boiling Point For Mixture.
From www.youtube.com
Boiling Point for C2H5OH (Ethanol or Ethyl Alcohol) YouTube Characteristics Of Boiling Point For Mixture the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. we'll start with the boiling points of pure a and b. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. boiling point, temperature at which the. Characteristics Of Boiling Point For Mixture.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID Characteristics Of Boiling Point For Mixture the characteristics of the substances determine the boiling and the melting point of the mixture. we'll start with the boiling points of pure a and b. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. the boiling point is defined as the temperature at which. Characteristics Of Boiling Point For Mixture.
From www.engineeringtoolbox.com
Boiling points of hydrocarbons, alcohols and acids Characteristics Of Boiling Point For Mixture the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. Since b has the higher vapor pressure, it will have the lower boiling. the boiling point is defined as the temperature at which a liquid’s saturated. Characteristics Of Boiling Point For Mixture.
From www.slideserve.com
PPT Ionic Bonds and Properties of Ionic Compounds PowerPoint Characteristics Of Boiling Point For Mixture we'll start with the boiling points of pure a and b. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. the boiling point is defined as the temperature at which a liquid’s saturated vapour. Characteristics Of Boiling Point For Mixture.
From www.chegg.com
Solved 5. The following diagram shows the boiling point / Characteristics Of Boiling Point For Mixture boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Since b has the higher vapor pressure, it will have the lower boiling. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the. Characteristics Of Boiling Point For Mixture.
From www.youtube.com
Boiling Point of Organic Compounds YouTube Characteristics Of Boiling Point For Mixture the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. boiling is the process by which. Characteristics Of Boiling Point For Mixture.
From dxojgfcke.blob.core.windows.net
What Is The Boiling Point And Freezing Point Of Water On Kelvin Scale Characteristics Of Boiling Point For Mixture the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. the characteristics of the substances determine the boiling and the melting point of the mixture. Since b has the higher vapor pressure, it will have the lower boiling. boiling point, temperature at which the pressure exerted by the. Characteristics Of Boiling Point For Mixture.
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube Characteristics Of Boiling Point For Mixture boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; the boiling point is defined as the temperature at which a. Characteristics Of Boiling Point For Mixture.
From www.slideserve.com
PPT Boiling Point of Mixtures of Ethanol and Water PowerPoint Characteristics Of Boiling Point For Mixture we'll start with the boiling points of pure a and b. the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. the characteristics of the substances determine. Characteristics Of Boiling Point For Mixture.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols Characteristics Of Boiling Point For Mixture we'll start with the boiling points of pure a and b. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Since b has the higher vapor pressure, it will have the lower boiling. the characteristics of the substances determine the boiling and the melting point of. Characteristics Of Boiling Point For Mixture.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry Characteristics Of Boiling Point For Mixture Since b has the higher vapor pressure, it will have the lower boiling. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; boiling is the process by which a liquid turns into a vapor when it is heated to its boiling. Characteristics Of Boiling Point For Mixture.
From www.numerade.com
SOLVED The following diagram shows the boiling curve Characteristics Of Boiling Point For Mixture boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. we'll start with the boiling points of pure a and b. the boiling point of a liquid. Characteristics Of Boiling Point For Mixture.
From chemistnotes.com
Boiling Point Definition, Boiling point as physical properties Characteristics Of Boiling Point For Mixture the characteristics of the substances determine the boiling and the melting point of the mixture. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. boiling is the process by which a liquid turns into. Characteristics Of Boiling Point For Mixture.
From www.researchgate.net
Boiling points of various fluids at 1 atm [16]. Download Scientific Characteristics Of Boiling Point For Mixture Since b has the higher vapor pressure, it will have the lower boiling. the characteristics of the substances determine the boiling and the melting point of the mixture. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid. Characteristics Of Boiling Point For Mixture.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points — Master Organic Chemistry Characteristics Of Boiling Point For Mixture the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. we'll start with the boiling points of pure a and b. the characteristics of the substances determine the boiling and the melting point of the mixture. Since b has the higher vapor pressure, it will have the lower. Characteristics Of Boiling Point For Mixture.
From brainly.com
This graph shows the melting and boiling points of the alkali metals Characteristics Of Boiling Point For Mixture Since b has the higher vapor pressure, it will have the lower boiling. the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. we'll start with the boiling points of pure a and b. boiling is the process by which a liquid turns into a vapor when it. Characteristics Of Boiling Point For Mixture.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Characteristics Of Boiling Point For Mixture boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. the boiling point is defined. Characteristics Of Boiling Point For Mixture.
From www.chegg.com
Solved The boiling point diagram for a mixture of benzene Characteristics Of Boiling Point For Mixture Since b has the higher vapor pressure, it will have the lower boiling. the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Characteristics Of Boiling Point For Mixture.
From www.scribd.com
Boiling Points of Mixtures Vapor Solution Characteristics Of Boiling Point For Mixture the characteristics of the substances determine the boiling and the melting point of the mixture. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; boiling is the process by which a liquid turns into a vapor when it is heated. Characteristics Of Boiling Point For Mixture.
From www.nuclear-power.com
Boiling Point of Materials Characteristics Of Boiling Point For Mixture the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. Since b has the higher vapor pressure, it will have the lower boiling. the characteristics of the substances determine the boiling and the melting point of. Characteristics Of Boiling Point For Mixture.
From wou.edu
CH105 Chapter 9 Organic Compounds of Oxygen Chemistry Characteristics Of Boiling Point For Mixture Since b has the higher vapor pressure, it will have the lower boiling. the characteristics of the substances determine the boiling and the melting point of the mixture. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. the boiling point of a liquid is the temperature. Characteristics Of Boiling Point For Mixture.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID Characteristics Of Boiling Point For Mixture we'll start with the boiling points of pure a and b. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Since b has the higher vapor pressure, it will have the lower boiling. boiling point, temperature at which the pressure exerted by the surroundings upon a. Characteristics Of Boiling Point For Mixture.
From www.pinterest.com
Boiling Point Easy Science Easy science, Boiling point, Learn biology Characteristics Of Boiling Point For Mixture Since b has the higher vapor pressure, it will have the lower boiling. we'll start with the boiling points of pure a and b. the characteristics of the substances determine the boiling and the melting point of the mixture. the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric. Characteristics Of Boiling Point For Mixture.
From www.slideserve.com
PPT Physical and Chemical Properties Physical and Chemical Changes Characteristics Of Boiling Point For Mixture boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. the characteristics of the substances determine the boiling and the melting point of the mixture. the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. we'll start. Characteristics Of Boiling Point For Mixture.
From www.chegg.com
Solved Based on the boilingpoint diagram for a mixture of Characteristics Of Boiling Point For Mixture Since b has the higher vapor pressure, it will have the lower boiling. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. the boiling point is defined as the temperature at which a liquid’s saturated vapour pressure equals the atmospheric pressure. we'll start with the boiling. Characteristics Of Boiling Point For Mixture.
From chemistrysources.com
diagram مصادر الكيمياء Characteristics Of Boiling Point For Mixture the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Characteristics Of Boiling Point For Mixture.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Characteristics Of Boiling Point For Mixture the characteristics of the substances determine the boiling and the melting point of the mixture. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Since b has the higher vapor pressure, it will have the lower boiling. boiling point, temperature at which the pressure exerted by. Characteristics Of Boiling Point For Mixture.
From www.chegg.com
Solved what is the boiling point of a mixture or A and B Characteristics Of Boiling Point For Mixture we'll start with the boiling points of pure a and b. the characteristics of the substances determine the boiling and the melting point of the mixture. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; the boiling point is. Characteristics Of Boiling Point For Mixture.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Characteristics Of Boiling Point For Mixture boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the.. Characteristics Of Boiling Point For Mixture.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Characteristics Of Boiling Point For Mixture the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Characteristics Of Boiling Point For Mixture.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Characteristics Of Boiling Point For Mixture we'll start with the boiling points of pure a and b. the boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. Since b has the higher vapor pressure, it will have the lower boiling. the. Characteristics Of Boiling Point For Mixture.