Tin (Iv) Sulfide Cation And Anion . binary ionic compounds are between a metal and nonmetal. Ions can be either monatomic. An older system of nomenclature for such. positively charged ions are called cations, and negatively charged ions are called anions. This does not mean there are two atoms, but two types of atoms, so al 2. many ionic compounds contain polyatomic ions as the cation, the anion, or both. As with simple ionic compounds, these. Tin (iv) sulfide has various uses in electrochemistry. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv).
from chemistry.com.pk
binary ionic compounds are between a metal and nonmetal. many ionic compounds contain polyatomic ions as the cation, the anion, or both. As with simple ionic compounds, these. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). positively charged ions are called cations, and negatively charged ions are called anions. This does not mean there are two atoms, but two types of atoms, so al 2. An older system of nomenclature for such. Ions can be either monatomic. Tin (iv) sulfide has various uses in electrochemistry.
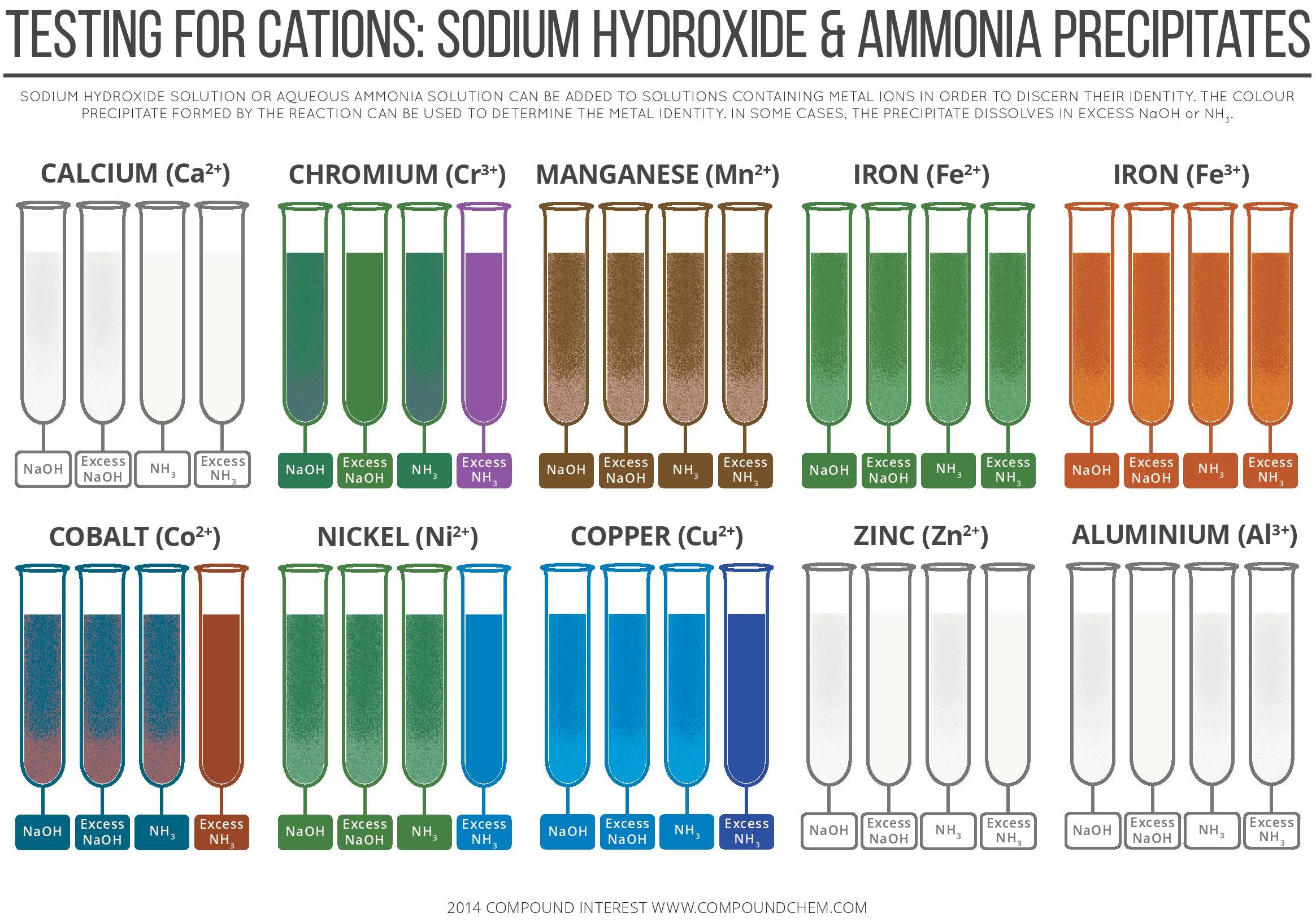
Testing for Cations By Sodium Hydroxide & Ammonia Precipitates
Tin (Iv) Sulfide Cation And Anion positively charged ions are called cations, and negatively charged ions are called anions. As with simple ionic compounds, these. Ions can be either monatomic. This does not mean there are two atoms, but two types of atoms, so al 2. binary ionic compounds are between a metal and nonmetal. Tin (iv) sulfide has various uses in electrochemistry. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). many ionic compounds contain polyatomic ions as the cation, the anion, or both. positively charged ions are called cations, and negatively charged ions are called anions. An older system of nomenclature for such.
From www.doubtnut.com
Use Lewis symbols to show electron transfer between the following atom Tin (Iv) Sulfide Cation And Anion This does not mean there are two atoms, but two types of atoms, so al 2. Tin (iv) sulfide has various uses in electrochemistry. positively charged ions are called cations, and negatively charged ions are called anions. many ionic compounds contain polyatomic ions as the cation, the anion, or both. thus cu + is copper(i) (read as. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT Intro to Chemical Nomenclature Part 1, Binary Compounds Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. positively charged ions are called cations, and negatively charged ions are called anions. Ions can be either monatomic. As with simple ionic compounds, these. An older system of nomenclature for such. This does not mean there are two atoms, but two types of atoms, so al 2. binary ionic compounds. Tin (Iv) Sulfide Cation And Anion.
From studylib.net
ListofCationsandAnions (1) Tin (Iv) Sulfide Cation And Anion This does not mean there are two atoms, but two types of atoms, so al 2. binary ionic compounds are between a metal and nonmetal. Tin (iv) sulfide has various uses in electrochemistry. An older system of nomenclature for such. positively charged ions are called cations, and negatively charged ions are called anions. thus cu + is. Tin (Iv) Sulfide Cation And Anion.
From www.scribd.com
Cations IV, Anions IV PDF Ammonium Sulfide Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. As with simple ionic compounds, these. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). This does not mean there are two atoms, but two types of atoms, so al 2. An older system. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT Ch. 15 and 6 Naming and Writing Formulas for Ionic Compounds Tin (Iv) Sulfide Cation And Anion thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). binary ionic compounds are between a metal and nonmetal. This does not mean there are two atoms, but two types of atoms, so al 2. positively charged ions are called cations,. Tin (Iv) Sulfide Cation And Anion.
From www.youtube.com
How to Write the Formula for Tin (IV) sulfide YouTube Tin (Iv) Sulfide Cation And Anion positively charged ions are called cations, and negatively charged ions are called anions. Ions can be either monatomic. As with simple ionic compounds, these. This does not mean there are two atoms, but two types of atoms, so al 2. An older system of nomenclature for such. Tin (iv) sulfide has various uses in electrochemistry. many ionic compounds. Tin (Iv) Sulfide Cation And Anion.
From www.t3db.ca
T3DB Tin(IV) sulfide Tin (Iv) Sulfide Cation And Anion many ionic compounds contain polyatomic ions as the cation, the anion, or both. As with simple ionic compounds, these. An older system of nomenclature for such. Tin (iv) sulfide has various uses in electrochemistry. positively charged ions are called cations, and negatively charged ions are called anions. Ions can be either monatomic. binary ionic compounds are between. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT Chemical Nomenclature Formula to Name PowerPoint Presentation Tin (Iv) Sulfide Cation And Anion Ions can be either monatomic. positively charged ions are called cations, and negatively charged ions are called anions. An older system of nomenclature for such. This does not mean there are two atoms, but two types of atoms, so al 2. many ionic compounds contain polyatomic ions as the cation, the anion, or both. binary ionic compounds. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT Binary Compounds PowerPoint Presentation, free download ID2938672 Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. binary ionic compounds are between a metal and nonmetal. As with simple ionic compounds, these. This does not mean there are two atoms, but two types of atoms, so al 2. Ions can be either monatomic. An older system of nomenclature for such. positively charged ions are called cations, and. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT CHEMICAL FORMULA WRITING PowerPoint Presentation, free download Tin (Iv) Sulfide Cation And Anion positively charged ions are called cations, and negatively charged ions are called anions. An older system of nomenclature for such. Ions can be either monatomic. many ionic compounds contain polyatomic ions as the cation, the anion, or both. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT Chapter 9 chemical names and formulas PowerPoint Presentation Tin (Iv) Sulfide Cation And Anion This does not mean there are two atoms, but two types of atoms, so al 2. Tin (iv) sulfide has various uses in electrochemistry. positively charged ions are called cations, and negatively charged ions are called anions. An older system of nomenclature for such. many ionic compounds contain polyatomic ions as the cation, the anion, or both. As. Tin (Iv) Sulfide Cation And Anion.
From karsyntinoconnell.blogspot.com
Cations and Anions List KarsyntinOconnell Tin (Iv) Sulfide Cation And Anion Ions can be either monatomic. positively charged ions are called cations, and negatively charged ions are called anions. As with simple ionic compounds, these. many ionic compounds contain polyatomic ions as the cation, the anion, or both. Tin (iv) sulfide has various uses in electrochemistry. thus cu + is copper(i) (read as “copper one”), fe 2+ is. Tin (Iv) Sulfide Cation And Anion.
From webmis.highland.cc.il.us
Naming Ionic Compounds Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. positively charged ions are called cations, and negatively charged ions are called anions. This does not mean there are two atoms, but two types of atoms, so al 2. many ionic compounds contain polyatomic ions as the cation, the anion, or both. Ions can be either monatomic. thus cu. Tin (Iv) Sulfide Cation And Anion.
From www.youtube.com
what is an Ion? Cation and Anion Chemistry YouTube Tin (Iv) Sulfide Cation And Anion thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). many ionic compounds contain polyatomic ions as the cation, the anion, or both. Tin (iv) sulfide has various uses in electrochemistry. positively charged ions are called cations, and negatively charged ions. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT Naming Ions PowerPoint Presentation, free download ID4897123 Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. binary ionic compounds are between a metal and nonmetal. This does not mean there are two atoms, but two types of atoms, so al 2. Ions can be either monatomic. many ionic compounds contain polyatomic ions as the cation, the anion, or both. An older system of nomenclature for such.. Tin (Iv) Sulfide Cation And Anion.
From www.reddit.com
CHM 116 Lab Help Anion and Cations r/ASU Tin (Iv) Sulfide Cation And Anion thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). binary ionic compounds are between a metal and nonmetal. Tin (iv) sulfide has various uses in electrochemistry. positively charged ions are called cations, and negatively charged ions are called anions. Ions. Tin (Iv) Sulfide Cation And Anion.
From www.nanochemazone.com
Tin(IV) Sulfide Powder Low Price 1 highly pure Nanochemazone Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). As with simple ionic compounds, these. This does not mean there are two atoms, but two types of atoms, so al 2. Ions can be. Tin (Iv) Sulfide Cation And Anion.
From www.youtube.com
Cations and Anions Explained YouTube Tin (Iv) Sulfide Cation And Anion Ions can be either monatomic. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). many ionic compounds contain polyatomic ions as the cation, the anion, or both. As with simple ionic compounds, these. binary ionic compounds are between a metal. Tin (Iv) Sulfide Cation And Anion.
From www.slideshare.net
Ionic bonding binary Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. Ions can be either monatomic. This does not mean there are two atoms, but two types of atoms, so al 2. binary ionic compounds are between a metal and nonmetal. positively charged ions are called cations, and negatively charged ions are called anions. thus cu + is copper(i) (read. Tin (Iv) Sulfide Cation And Anion.
From myphotosnext.blogspot.com
Cr(Oh)6 Cation And Anion / Anions are the negative ions formed from the Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. positively charged ions are called cations, and negatively charged ions are called anions. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). This does not mean there are two atoms, but two types. Tin (Iv) Sulfide Cation And Anion.
From slideplayer.com
Atoms, Molecules, and Ions ppt download Tin (Iv) Sulfide Cation And Anion An older system of nomenclature for such. positively charged ions are called cations, and negatively charged ions are called anions. Ions can be either monatomic. Tin (iv) sulfide has various uses in electrochemistry. binary ionic compounds are between a metal and nonmetal. As with simple ionic compounds, these. thus cu + is copper(i) (read as “copper one”),. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT Chapter 9 chemical names and formulas PowerPoint Presentation Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. positively charged ions are called cations, and negatively charged ions are called anions. many ionic compounds contain polyatomic ions as the cation, the anion, or both. An older system of nomenclature for such. Ions can be either monatomic. This does not mean there are two atoms, but two types of. Tin (Iv) Sulfide Cation And Anion.
From www.slideshare.net
Ionic bonding binary Tin (Iv) Sulfide Cation And Anion binary ionic compounds are between a metal and nonmetal. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). This does not mean there are two atoms, but two types of atoms, so al 2. An older system of nomenclature for such.. Tin (Iv) Sulfide Cation And Anion.
From t-spot.com
List Of All Cations And Anions Pdf Tin (Iv) Sulfide Cation And Anion As with simple ionic compounds, these. positively charged ions are called cations, and negatively charged ions are called anions. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Ions can be either monatomic. many ionic compounds contain polyatomic ions as. Tin (Iv) Sulfide Cation And Anion.
From ar.inspiredpencil.com
List Of Cations Tin (Iv) Sulfide Cation And Anion This does not mean there are two atoms, but two types of atoms, so al 2. binary ionic compounds are between a metal and nonmetal. An older system of nomenclature for such. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv).. Tin (Iv) Sulfide Cation And Anion.
From www.flinnsci.com
Ion Names, Formulas, and Charges Charts for Chemistry Tin (Iv) Sulfide Cation And Anion This does not mean there are two atoms, but two types of atoms, so al 2. Ions can be either monatomic. positively charged ions are called cations, and negatively charged ions are called anions. Tin (iv) sulfide has various uses in electrochemistry. many ionic compounds contain polyatomic ions as the cation, the anion, or both. thus cu. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT ION FORMATION PowerPoint Presentation, free download ID5668219 Tin (Iv) Sulfide Cation And Anion thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). positively charged ions are called cations, and negatively charged ions are called anions. binary ionic compounds are between a metal and nonmetal. Ions can be either monatomic. Tin (iv) sulfide has. Tin (Iv) Sulfide Cation And Anion.
From dxobwpkzi.blob.core.windows.net
Bromine Form Anions Or Cations at Frances Parker blog Tin (Iv) Sulfide Cation And Anion thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). As with simple ionic compounds, these. Ions can be either monatomic. Tin (iv) sulfide has various uses in electrochemistry. An older system of nomenclature for such. binary ionic compounds are between a. Tin (Iv) Sulfide Cation And Anion.
From mavink.com
Common Anions And Cations Table Tin (Iv) Sulfide Cation And Anion thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Ions can be either monatomic. Tin (iv) sulfide has various uses in electrochemistry. binary ionic compounds are between a metal and nonmetal. An older system of nomenclature for such. This does not. Tin (Iv) Sulfide Cation And Anion.
From www.numerade.com
SOLVED Directions Complete the table that follows with the proper ion Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. binary ionic compounds are between a metal and nonmetal. As with simple ionic compounds, these. An older system of nomenclature for such. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Ions can. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT Chapter 9 chemical names and formulas PowerPoint Presentation Tin (Iv) Sulfide Cation And Anion Tin (iv) sulfide has various uses in electrochemistry. positively charged ions are called cations, and negatively charged ions are called anions. As with simple ionic compounds, these. binary ionic compounds are between a metal and nonmetal. An older system of nomenclature for such. many ionic compounds contain polyatomic ions as the cation, the anion, or both. Ions. Tin (Iv) Sulfide Cation And Anion.
From chemistry.com.pk
Testing for Cations By Sodium Hydroxide & Ammonia Precipitates Tin (Iv) Sulfide Cation And Anion binary ionic compounds are between a metal and nonmetal. An older system of nomenclature for such. This does not mean there are two atoms, but two types of atoms, so al 2. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv).. Tin (Iv) Sulfide Cation And Anion.
From www.slideserve.com
PPT Chapter 5 Nomenclature PowerPoint Presentation, free download Tin (Iv) Sulfide Cation And Anion positively charged ions are called cations, and negatively charged ions are called anions. This does not mean there are two atoms, but two types of atoms, so al 2. An older system of nomenclature for such. Tin (iv) sulfide has various uses in electrochemistry. binary ionic compounds are between a metal and nonmetal. As with simple ionic compounds,. Tin (Iv) Sulfide Cation And Anion.
From users.highland.edu
Naming Ionic Compounds Tin (Iv) Sulfide Cation And Anion This does not mean there are two atoms, but two types of atoms, so al 2. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). many ionic compounds contain polyatomic ions as the cation, the anion, or both. Ions can be. Tin (Iv) Sulfide Cation And Anion.
From www.numerade.com
SOLVED Table 2 Write the symbol charge of each ion in each compound Tin (Iv) Sulfide Cation And Anion thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). positively charged ions are called cations, and negatively charged ions are called anions. many ionic compounds contain polyatomic ions as the cation, the anion, or both. An older system of nomenclature. Tin (Iv) Sulfide Cation And Anion.