Vitamin C Content In Orange Juice Experiment . The best samples are lightly colored and/or easily pulverized. Well, wonder no longer, with this titration experiment students discover how much, or little,. To investigate the vitamin c content in different fruit juices. Measure the vitamin c content of a sample of fruit juice by measuring the volume of the sample required to decolourise a solution of dcpip. The two reactions we will use in this experiment are: The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). Adding tincture of iodine (top right) 3. How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? Adding orange juice until the dark colour disappears. Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? This procedure can be used to. Adding starch solution (middle left) 4. Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq).
from parasitic-diseases-examples.blogspot.com
Measure the vitamin c content of a sample of fruit juice by measuring the volume of the sample required to decolourise a solution of dcpip. The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). The best samples are lightly colored and/or easily pulverized. To investigate the vitamin c content in different fruit juices. Adding tincture of iodine (top right) 3. Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq). Adding starch solution (middle left) 4. Well, wonder no longer, with this titration experiment students discover how much, or little,. Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration?
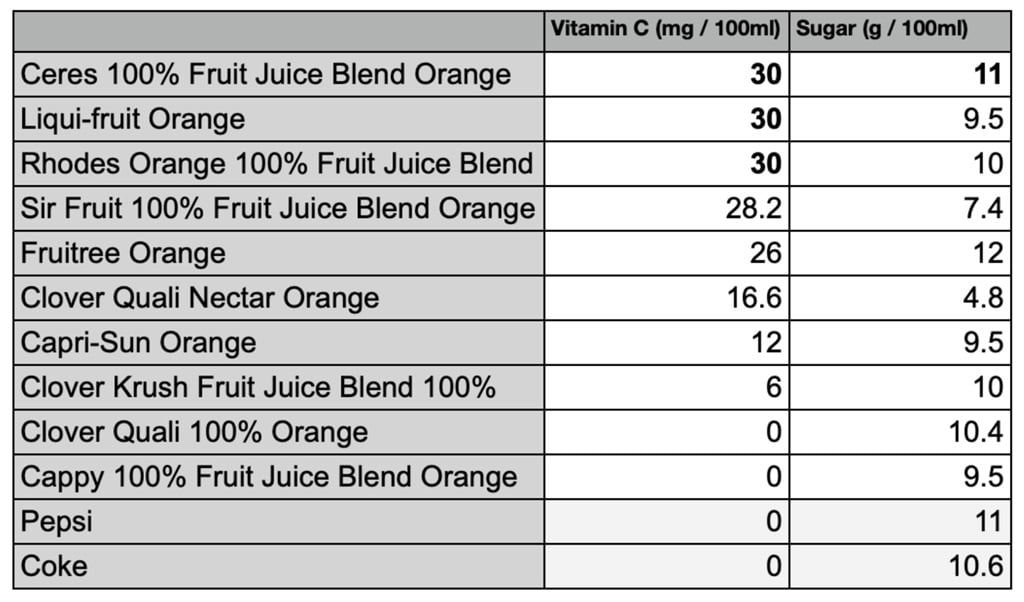
Some orange juice in SA has more sugar than Coke and about as much
Vitamin C Content In Orange Juice Experiment Well, wonder no longer, with this titration experiment students discover how much, or little,. Adding starch solution (middle left) 4. Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq). The best samples are lightly colored and/or easily pulverized. Measure the vitamin c content of a sample of fruit juice by measuring the volume of the sample required to decolourise a solution of dcpip. This procedure can be used to. Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? The two reactions we will use in this experiment are: To investigate the vitamin c content in different fruit juices. Adding orange juice until the dark colour disappears. How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). Well, wonder no longer, with this titration experiment students discover how much, or little,. Adding tincture of iodine (top right) 3.
From www.researchgate.net
Effect of time on the vitamin C content of orange juices stored at 4 Vitamin C Content In Orange Juice Experiment Well, wonder no longer, with this titration experiment students discover how much, or little,. Adding orange juice until the dark colour disappears. Measure the vitamin c content of a sample of fruit juice by measuring the volume of the sample required to decolourise a solution of dcpip. Adding tincture of iodine (top right) 3. To investigate the vitamin c content. Vitamin C Content In Orange Juice Experiment.
From www.slideserve.com
PPT VITAMIN C CONCENTRATION IN DIFFERENT ORANGE JUICE BRANDS Vitamin C Content In Orange Juice Experiment Measure the vitamin c content of a sample of fruit juice by measuring the volume of the sample required to decolourise a solution of dcpip. Adding starch solution (middle left) 4. Well, wonder no longer, with this titration experiment students discover how much, or little,. The two reactions we will use in this experiment are: Adding orange juice until the. Vitamin C Content In Orange Juice Experiment.
From tinhte.vn
[Infographic] 9 loại rau quả giàu vitamin C hơn cam Tinhte.vn Vitamin C Content In Orange Juice Experiment Well, wonder no longer, with this titration experiment students discover how much, or little,. This procedure can be used to. Measure the vitamin c content of a sample of fruit juice by measuring the volume of the sample required to decolourise a solution of dcpip. Adding starch solution (middle left) 4. How does increasing the temperature of orange juice affect. Vitamin C Content In Orange Juice Experiment.
From www.chegg.com
Solved Experiment 4 Determination of Vitamin C in Fruit Vitamin C Content In Orange Juice Experiment Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? Adding orange juice until the dark colour disappears. The two reactions we will use in this experiment are: To investigate the vitamin c content in different fruit juices. The best samples are lightly colored and/or easily pulverized. Measure the vitamin. Vitamin C Content In Orange Juice Experiment.
From www.slideshare.net
analysis vitamin c in commercial fruit juice Vitamin C Content In Orange Juice Experiment Measure the vitamin c content of a sample of fruit juice by measuring the volume of the sample required to decolourise a solution of dcpip. The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). The best samples are lightly colored and/or easily. Vitamin C Content In Orange Juice Experiment.
From www.all-science-fair-projects.com
Vitamin C in Orange Juice Science Fair Projects STEM Projects Vitamin C Content In Orange Juice Experiment The two reactions we will use in this experiment are: To investigate the vitamin c content in different fruit juices. How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq). Adding tincture of iodine (top. Vitamin C Content In Orange Juice Experiment.
From www.thinkswap.com
Vitamin C Content of Orange Juice Nutrition Year 12 SACE Thinkswap Vitamin C Content In Orange Juice Experiment Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq). The two reactions we will use in this experiment are: Well, wonder no longer, with this titration experiment students discover how much, or little,. The best samples are lightly colored and/or easily pulverized. Have you ever poured a tall glass. Vitamin C Content In Orange Juice Experiment.
From slidetodoc.com
VITAMIN C CONCENTRATION IN DIFFERENT ORANGE JUICE BRANDS Vitamin C Content In Orange Juice Experiment To investigate the vitamin c content in different fruit juices. The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). Well, wonder no longer, with this titration experiment students discover how much, or little,. Kio 3(aq) + 6h + (aq) + 5i −. Vitamin C Content In Orange Juice Experiment.
From plotly.com
A graph to show the Vitamin C content of different fruit juices as Vitamin C Content In Orange Juice Experiment Adding starch solution (middle left) 4. The two reactions we will use in this experiment are: The best samples are lightly colored and/or easily pulverized. Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq). How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration?. Vitamin C Content In Orange Juice Experiment.
From www.slideserve.com
PPT VITAMIN C CONCENTRATION IN DIFFERENT ORANGE JUICE BRANDS Vitamin C Content In Orange Juice Experiment Well, wonder no longer, with this titration experiment students discover how much, or little,. Measure the vitamin c content of a sample of fruit juice by measuring the volume of the sample required to decolourise a solution of dcpip. This procedure can be used to. The two reactions we will use in this experiment are: Adding starch solution (middle left). Vitamin C Content In Orange Juice Experiment.
From parasitic-diseases-examples.blogspot.com
Some orange juice in SA has more sugar than Coke and about as much Vitamin C Content In Orange Juice Experiment The two reactions we will use in this experiment are: The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). Adding starch solution (middle left) 4. Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin. Vitamin C Content In Orange Juice Experiment.
From www.slideshare.net
Vitamin c in orange juices Vitamin C Content In Orange Juice Experiment Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? The two reactions we will use in this experiment are: Adding tincture of iodine (top right) 3. Adding orange juice until the dark colour disappears. This procedure can be used to. How does increasing the temperature of orange juice affect. Vitamin C Content In Orange Juice Experiment.
From www.savemyexams.com
Practical Vitamin C Content (1.2.11) Edexcel A (SNAB) A Level Vitamin C Content In Orange Juice Experiment This procedure can be used to. Adding tincture of iodine (top right) 3. The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). The two reactions we will use in this experiment are: Measure the vitamin c content of a sample of fruit. Vitamin C Content In Orange Juice Experiment.
From www.slideshare.net
Vitamin c content Vitamin C Content In Orange Juice Experiment Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? This procedure can be used to. The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). The best samples are lightly colored and/or. Vitamin C Content In Orange Juice Experiment.
From coleenenglish.blogspot.com
41 does storage temperature affect orange juice acidity Online Education Vitamin C Content In Orange Juice Experiment Well, wonder no longer, with this titration experiment students discover how much, or little,. Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq). Adding starch solution (middle left) 4. How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? The concentration of vitamin c. Vitamin C Content In Orange Juice Experiment.
From www.flinnsci.com
Vitamin C Analysis Fruit and Fruit Juices—ChemTopic™ Lab Activity Vitamin C Content In Orange Juice Experiment Adding tincture of iodine (top right) 3. How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? The two reactions we will use in this experiment are: To investigate the vitamin c content in different fruit juices. Adding orange juice until the dark colour disappears. Measure the vitamin c content of a sample of fruit. Vitamin C Content In Orange Juice Experiment.
From www.slideshare.net
analysis vitamin c in commercial fruit juice Vitamin C Content In Orange Juice Experiment Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq). This procedure can be used to. Adding orange juice until the dark colour disappears. How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? Measure the vitamin c content of a sample of fruit juice. Vitamin C Content In Orange Juice Experiment.
From thehomeschoolscientist.com
Testing for Vitamin C with Iodine The Homeschool Scientist Vitamin C Content In Orange Juice Experiment The two reactions we will use in this experiment are: To investigate the vitamin c content in different fruit juices. This procedure can be used to. Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq). The concentration of vitamin c in a fruit juice can be determined using a. Vitamin C Content In Orange Juice Experiment.
From giopvflkk.blob.core.windows.net
How To Calculate Vitamin C In Orange Juice at Josie Green blog Vitamin C Content In Orange Juice Experiment The best samples are lightly colored and/or easily pulverized. Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq). Adding tincture of iodine (top right) 3. This procedure can be used to. Adding starch solution (middle left) 4. The two reactions we will use in this experiment are: To investigate. Vitamin C Content In Orange Juice Experiment.
From www.slideserve.com
PPT VITAMIN C CONCENTRATION IN DIFFERENT ORANGE JUICE BRANDS Vitamin C Content In Orange Juice Experiment The two reactions we will use in this experiment are: Adding starch solution (middle left) 4. The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). Measure the vitamin c content of a sample of fruit juice by measuring the volume of the. Vitamin C Content In Orange Juice Experiment.
From www.coursehero.com
[Solved] Part III. Determine the content of Vit. C from the average Vitamin C Content In Orange Juice Experiment Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? The best samples are lightly colored and/or easily pulverized. The two reactions we will use in this experiment are: Well, wonder no longer, with this titration experiment students discover how much, or little,. To investigate the vitamin c content in. Vitamin C Content In Orange Juice Experiment.
From www.slideshare.net
Vitamin c in orange juices Vitamin C Content In Orange Juice Experiment Adding tincture of iodine (top right) 3. This procedure can be used to. Well, wonder no longer, with this titration experiment students discover how much, or little,. Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? To investigate the vitamin c content in different fruit juices. The two reactions. Vitamin C Content In Orange Juice Experiment.
From www.slideserve.com
PPT VITAMIN C CONCENTRATION IN DIFFERENT ORANGE JUICE BRANDS Vitamin C Content In Orange Juice Experiment Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) + 3h 2o(l) + k + (aq). How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? The two reactions we will use in this experiment are: This procedure can be used to. Adding starch solution (middle left) 4. The best samples. Vitamin C Content In Orange Juice Experiment.
From 9cbettycoco.weebly.com
Science Fair Which Package Orange Juice Contains the Most Vitamin C Vitamin C Content In Orange Juice Experiment Adding orange juice until the dark colour disappears. Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? The two reactions we will use in this experiment are: This procedure can be used to. Well, wonder no longer, with this titration experiment students discover how much, or little,. Kio 3(aq). Vitamin C Content In Orange Juice Experiment.
From melscience.com
Vitamin C MEL Chemistry Vitamin C Content In Orange Juice Experiment How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? The two reactions we will use in this experiment are: This procedure can be used to. The best samples are lightly colored and/or easily pulverized. To investigate the vitamin c content in different fruit juices. Have you ever poured a tall glass of a fruit. Vitamin C Content In Orange Juice Experiment.
From keplarllp.com
😎 Vitamin c content in orange juice experiment. The Comparison of Vitamin C Content In Orange Juice Experiment This procedure can be used to. Adding starch solution (middle left) 4. Adding orange juice until the dark colour disappears. The best samples are lightly colored and/or easily pulverized. Well, wonder no longer, with this titration experiment students discover how much, or little,. To investigate the vitamin c content in different fruit juices. Have you ever poured a tall glass. Vitamin C Content In Orange Juice Experiment.
From aijn.eu
The Micronutrients in Orange Juice AIJN European Fruit Juice Vitamin C Content In Orange Juice Experiment The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). The two reactions we will use in this experiment are: Adding starch solution (middle left) 4. How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? Kio 3(aq). Vitamin C Content In Orange Juice Experiment.
From www.thinkswap.com
Determining Vitamin C in Fruit Juices Presentation 65111 Chemistry Vitamin C Content In Orange Juice Experiment The best samples are lightly colored and/or easily pulverized. Measure the vitamin c content of a sample of fruit juice by measuring the volume of the sample required to decolourise a solution of dcpip. Adding orange juice until the dark colour disappears. To investigate the vitamin c content in different fruit juices. How does increasing the temperature of orange juice. Vitamin C Content In Orange Juice Experiment.
From snabbiology.co.uk
Measuring the Content of Vitamin C in Fruit Juice Snab Biology Vitamin C Content In Orange Juice Experiment Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? This procedure can be used to. Adding tincture of iodine (top right) 3. The best samples are lightly colored and/or easily pulverized. Well, wonder no longer, with this titration experiment students discover how much, or little,. Adding starch solution (middle. Vitamin C Content In Orange Juice Experiment.
From www.slideserve.com
PPT Real World Vitamin C Concentration and Total Acidity in Orange Vitamin C Content In Orange Juice Experiment The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? To investigate the vitamin c content in different fruit juices. Well, wonder no longer, with this titration experiment. Vitamin C Content In Orange Juice Experiment.
From www.semanticscholar.org
A COMPARATIVE ANALYSIS OF VITAMINC CONCENTRATION IN COMMERCIAL FRUIT Vitamin C Content In Orange Juice Experiment Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). Adding starch solution (middle left) 4. The two reactions we will use in. Vitamin C Content In Orange Juice Experiment.
From www.pinterest.co.uk
Science Stuff How much Vitamin C is in your fruit juice? Teaching Vitamin C Content In Orange Juice Experiment Well, wonder no longer, with this titration experiment students discover how much, or little,. Adding starch solution (middle left) 4. Adding orange juice until the dark colour disappears. Have you ever poured a tall glass of a fruit drink, and wondered just how much vitamin c it contains? Measure the vitamin c content of a sample of fruit juice by. Vitamin C Content In Orange Juice Experiment.
From www.researchgate.net
Vitamin C content of juice from different orange (Citrus sinensis Vitamin C Content In Orange Juice Experiment The best samples are lightly colored and/or easily pulverized. The two reactions we will use in this experiment are: The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). Kio 3(aq) + 6h + (aq) + 5i − (aq) → 3i 2(aq) +. Vitamin C Content In Orange Juice Experiment.
From coremymages.blogspot.com
Vitamin C Content In Fruit Juices Experiment Results Coremymages Vitamin C Content In Orange Juice Experiment The concentration of vitamin c in a fruit juice can be determined using a redox titration with iodine (i 2), or alternatively with potassiun iodate (kio 3). To investigate the vitamin c content in different fruit juices. How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? This procedure can be used to. Well, wonder. Vitamin C Content In Orange Juice Experiment.
From www.researchgate.net
Vitamin C content of juice from different orange (Citrus sinensis Vitamin C Content In Orange Juice Experiment Adding orange juice until the dark colour disappears. To investigate the vitamin c content in different fruit juices. The two reactions we will use in this experiment are: How does increasing the temperature of orange juice affect its vitamin c (ascorbic acid) concentration? Measure the vitamin c content of a sample of fruit juice by measuring the volume of the. Vitamin C Content In Orange Juice Experiment.