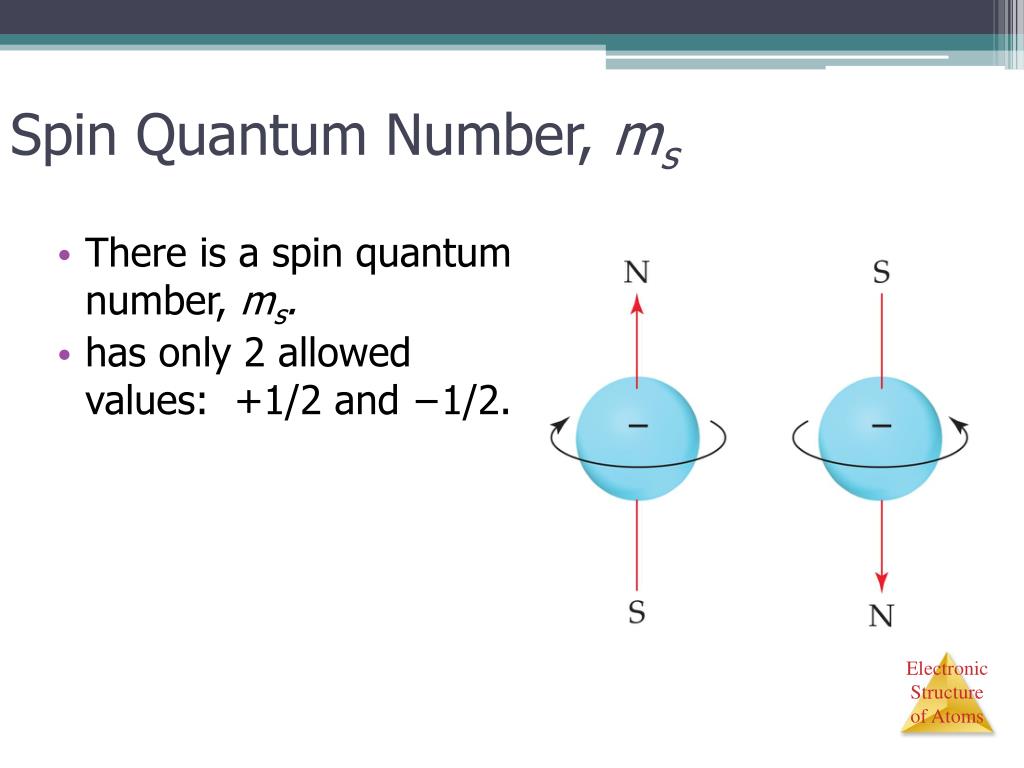
What Is The Spin Quantum Number . Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Learn about the history, models and.
from www.slideserve.com
The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn about the history, models and.
PPT Chapter 6 Electronic Structure of Atoms PowerPoint Presentation
What Is The Spin Quantum Number Learn about the history, models and. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Learn about the history, models and. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron.
From www.slideserve.com
PPT Quantum Numbers and Electronic Configuration PowerPoint What Is The Spin Quantum Number Learn about the history, models and. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in. What Is The Spin Quantum Number.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape What Is The Spin Quantum Number Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Learn about the history, models and. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Electron spin or spin. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Principle Quantum Numbers PowerPoint Presentation, free download What Is The Spin Quantum Number Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Learn about the history, models and. The spin quantum number (ms) represents the orientation of an electron's spin along a. What Is The Spin Quantum Number.
From slideplayer.com
Chapter 5 Quantum Numbers Part ppt download What Is The Spin Quantum Number The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn about the history, models and. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Electron. What Is The Spin Quantum Number.
From www.youtube.com
Spin Quantum Number I Atomic Structure I Particles Academy YouTube What Is The Spin Quantum Number Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. The spin quantum number (ms) represents. What Is The Spin Quantum Number.
From www.youtube.com
Spin Quantum NumberWhat is Spin Quantum Number Electron Spin What Is The Spin Quantum Number The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn about the history, models. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Chapter 6 Electronic Structure of Atoms PowerPoint Presentation What Is The Spin Quantum Number The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin quantum number is a property of electrons and other fermions that. What Is The Spin Quantum Number.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps What Is The Spin Quantum Number Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Spin is an. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Quantum Theory (cont.) 10 Sept. 2008 PowerPoint Presentation What Is The Spin Quantum Number Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Learn about the history, models and. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms. What Is The Spin Quantum Number.
From www.geeksforgeeks.org
Quantum Numbers Principal, Azimuthal, & Spin What Is The Spin Quantum Number The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn about the history, models and. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Learn how to use four. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Chapter 4 Arrangement of Electrons in Atoms PowerPoint What Is The Spin Quantum Number Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Spin quantum number is a property. What Is The Spin Quantum Number.
From education-portal.com
Spin Quantum Number Definition & Example Video & Lesson Transcript What Is The Spin Quantum Number Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn what the spin. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID2135857 What Is The Spin Quantum Number Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Learn about the history,. What Is The Spin Quantum Number.
From www.tes.com
Principle Quantum Number, Secondary Quantum Number, Spin Quantum What Is The Spin Quantum Number Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn about the history, models and. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Learn what the spin quantum number (s) is and how it describes the angular momentum. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Chapter Seven Atomic Structure PowerPoint Presentation, free What Is The Spin Quantum Number Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Spin is an intrinsic. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Spin Quantum Number, m s PowerPoint Presentation, free download What Is The Spin Quantum Number Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn about the history, models and. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Spin. What Is The Spin Quantum Number.
From ar.inspiredpencil.com
Spin Quantum Numbers What Is The Spin Quantum Number Learn about the history, models and. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Learn how to use. What Is The Spin Quantum Number.
From www.youtube.com
Quantum Numbers of Electrons (Principle, Azimuthal, Spin What Is The Spin Quantum Number Learn about the history, models and. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn what the spin quantum number (s) is and how it describes the angular momentum of. What Is The Spin Quantum Number.
From www.slideserve.com
PPT CHAPTER 5 PowerPoint Presentation, free download ID1709005 What Is The Spin Quantum Number Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Learn about the history, models and. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Spin quantum number is. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID4952601 What Is The Spin Quantum Number Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn what the spin quantum number (s) is and how it. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Chapter 28 PowerPoint Presentation, free download ID1104026 What Is The Spin Quantum Number Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn about the history, models and. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms.. What Is The Spin Quantum Number.
From deepstash.com
Spin Quantum Numbers (ms) Deepstash What Is The Spin Quantum Number The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Learn about the history, models and. Electron spin or spin quantum. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Quantum Numbers Activity PowerPoint Presentation, free download What Is The Spin Quantum Number The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Learn how to use four quantum numbers (principal, angular,. What Is The Spin Quantum Number.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin What Is The Spin Quantum Number Learn about the history, models and. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Spin Quantum Number, m s PowerPoint Presentation, free download What Is The Spin Quantum Number The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Learn about the history, models and. Electron spin or spin quantum. What Is The Spin Quantum Number.
From www.youtube.com
Class 11 chap 2 Spin Quantum Number Quantum Numbers JEE / NEET What Is The Spin Quantum Number Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn about the history, models and. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Learn what the spin quantum. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID834035 What Is The Spin Quantum Number Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin quantum number is a property of electrons and. What Is The Spin Quantum Number.
From www.vecteezy.com
Spin Quantum Number physics vector illustration infographic 26586277 What Is The Spin Quantum Number The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn what the spin quantum. What Is The Spin Quantum Number.
From www.chemistrylearner.com
Physical Chemistry Page 2 of 3 Chemistry Learner What Is The Spin Quantum Number Learn about the history, models and. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. Learn what the spin quantum number (s) is and how it describes the angular momentum of an. What Is The Spin Quantum Number.
From study.com
Quantum Numbers on the Periodic Table Definition & Overview Lesson What Is The Spin Quantum Number Spin is an intrinsic form of angular momentum carried by elementary particles and composite systems. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Learn about the history, models and. Learn how to use four quantum. What Is The Spin Quantum Number.
From www.slideserve.com
PPT The fourth quantum number Spin PowerPoint Presentation, free What Is The Spin Quantum Number Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Learn about the history, models and. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Unit 2 PowerPoint Presentation, free download ID298336 What Is The Spin Quantum Number Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Learn about the history, models and. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in. What Is The Spin Quantum Number.
From www.slideserve.com
PPT Quantum Number and Electron Configurations PowerPoint What Is The Spin Quantum Number Learn about the history, models and. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin is an. What Is The Spin Quantum Number.
From deepstash.com
Spin Quantum Numbers (ms) Deepstash What Is The Spin Quantum Number Learn about the history, models and. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Learn how to. What Is The Spin Quantum Number.
From www.chemistrylearner.com
Spin Quantum Number Definition, Significance, and Value What Is The Spin Quantum Number The spin quantum number (ms) represents the orientation of an electron's spin along a specific axis. Learn about the history, models and. Learn what the spin quantum number (s) is and how it describes the angular momentum of an electron. Spin quantum number is a property of electrons and other fermions that describes their intrinsic angular momentum. Electron spin or. What Is The Spin Quantum Number.