Bromine Ion Or Isotope . Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. Natural bromine is a mixture of two stable isotopes: It is defined as being the charge that an atom would have if all bonds were ionic. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. Accelerates the burning of combustible. 49.5 if you want to be fussy!). List, data and properties of all known isotopes of bromine. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Denser than water and soluble in water. That means that a compound containing 1 bromine atom will have two peaks in. Uncombined elements have an oxidation state of 0. All atomic nuclei of the chemical element bromine are summarized under.
from imsyaf.com
Uncombined elements have an oxidation state of 0. Natural bromine is a mixture of two stable isotopes: Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. All atomic nuclei of the chemical element bromine are summarized under. Accelerates the burning of combustible. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. 49.5 if you want to be fussy!). Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Denser than water and soluble in water. List, data and properties of all known isotopes of bromine.
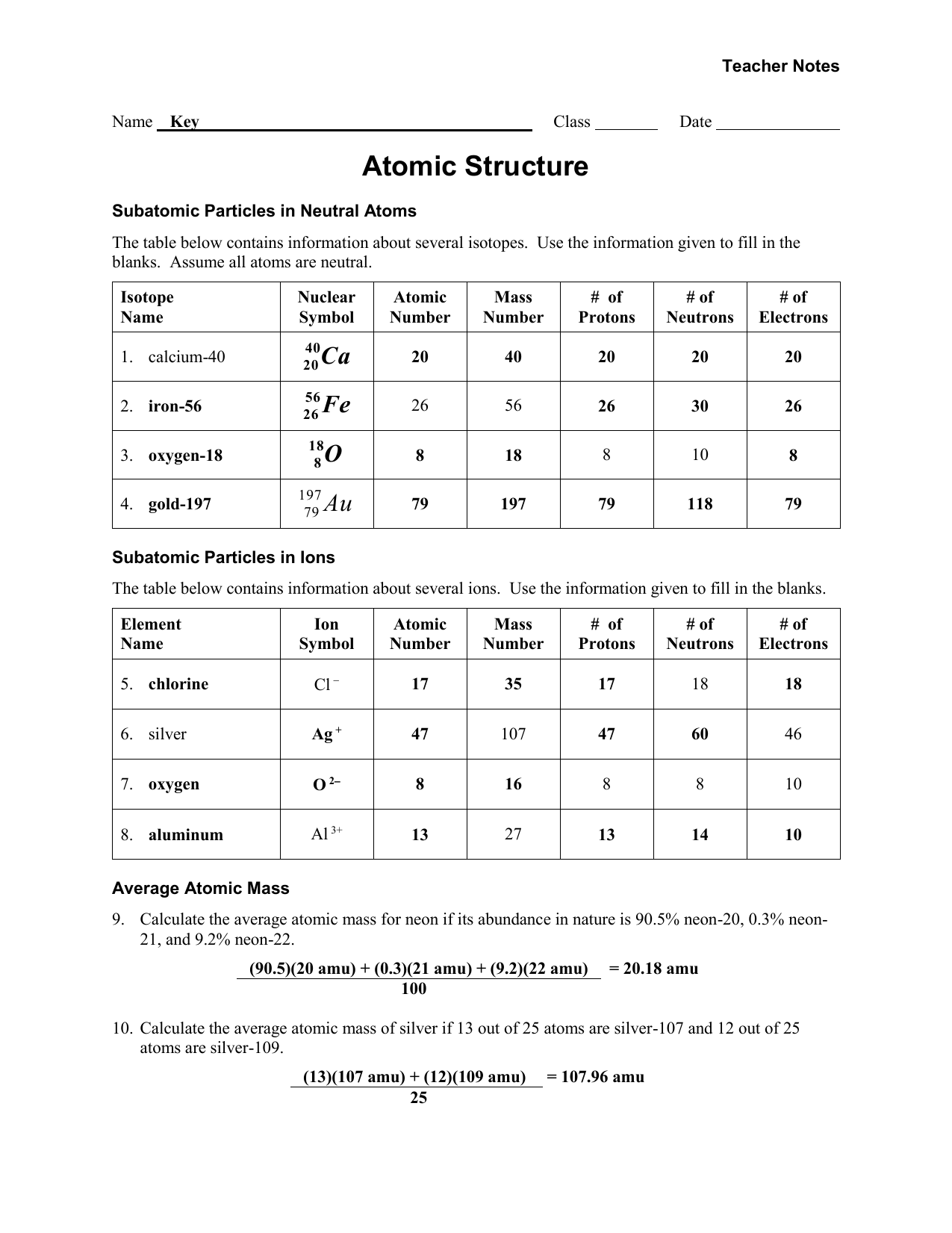
Isotopes Ions And Atoms Worksheet
Bromine Ion Or Isotope List, data and properties of all known isotopes of bromine. Denser than water and soluble in water. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. List, data and properties of all known isotopes of bromine. Natural bromine is a mixture of two stable isotopes: Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Accelerates the burning of combustible. Uncombined elements have an oxidation state of 0. That means that a compound containing 1 bromine atom will have two peaks in. It is defined as being the charge that an atom would have if all bonds were ionic. All atomic nuclei of the chemical element bromine are summarized under. Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. 49.5 if you want to be fussy!).
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Bromine Ion Or Isotope Denser than water and soluble in water. It is defined as being the charge that an atom would have if all bonds were ionic. Accelerates the burning of combustible. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. 49.5 if you want to be fussy!). Bromine has two isotopes, 79 br. Bromine Ion Or Isotope.
From www.chegg.com
Solved bromine isotope with 49 neutrons Express your answer Bromine Ion Or Isotope All atomic nuclei of the chemical element bromine are summarized under. That means that a compound containing 1 bromine atom will have two peaks in. List, data and properties of all known isotopes of bromine. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. It is defined as being the charge. Bromine Ion Or Isotope.
From www.buyisotope.com
81Br Isotope, Enriched 81Br, 81Br Sodium Bromide, 81Br Price Bromine Ion Or Isotope 49.5 if you want to be fussy!). Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were ionic. List, data and properties of all known isotopes of bromine. All atomic nuclei of the chemical element bromine are summarized under. This pattern of molecular ions is a. Bromine Ion Or Isotope.
From ceogwcrc.blob.core.windows.net
Bromine Isotopes Most Abundant at Milton Simmons blog Bromine Ion Or Isotope Natural bromine is a mixture of two stable isotopes: Denser than water and soluble in water. That means that a compound containing 1 bromine atom will have two peaks in. Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 :. Bromine Ion Or Isotope.
From giofiorxy.blob.core.windows.net
Chlorine And Bromine Isotopes at Linda Henry blog Bromine Ion Or Isotope Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : That means that a compound containing 1 bromine atom will have two peaks in. All atomic nuclei of the chemical element bromine are summarized under. Accelerates the burning of combustible. Denser than water and soluble in water. This pattern of molecular ions is a. Bromine Ion Or Isotope.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Ion Or Isotope List, data and properties of all known isotopes of bromine. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. It is defined as being the charge that an atom would have if all bonds were ionic. Accelerates the burning of combustible. Denser than water and soluble in water. Natural bromine is. Bromine Ion Or Isotope.
From www.numerade.com
SOLVEDBromine has two naturally occurring isotopes. One of them Bromine Ion Or Isotope This pattern of molecular ions is a good indication that there is a bromine present in the molecule. Natural bromine is a mixture of two stable isotopes: That means that a compound containing 1 bromine atom will have two peaks in. Denser than water and soluble in water. Similarly, chlorobenzene should display m+ at m/z =112, provided you take into. Bromine Ion Or Isotope.
From fyoxfvhok.blob.core.windows.net
Symbol For Bromine Oxide at Merle Dunn blog Bromine Ion Or Isotope Accelerates the burning of combustible. Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were ionic. List, data and properties of all known isotopes of bromine. That means that a compound containing. Bromine Ion Or Isotope.
From imgflip.com
Bromine Isotopic Abundance Imgflip Bromine Ion Or Isotope Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : It is defined as being the charge that an atom would have if all bonds were ionic. 49.5 if you want to be fussy!). All atomic nuclei of the chemical element bromine are summarized under. Denser than water and soluble in water. This pattern. Bromine Ion Or Isotope.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Ion Or Isotope Accelerates the burning of combustible. It is defined as being the charge that an atom would have if all bonds were ionic. 49.5 if you want to be fussy!). Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : That means that a compound containing 1 bromine atom will have two peaks in. Natural. Bromine Ion Or Isotope.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Ion Or Isotope 49.5 if you want to be fussy!). List, data and properties of all known isotopes of bromine. Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. All atomic nuclei of the chemical element bromine are summarized under. Uncombined elements have an oxidation state of 0. That means that a compound containing 1 bromine atom will. Bromine Ion Or Isotope.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The Bromine Ion Or Isotope List, data and properties of all known isotopes of bromine. Natural bromine is a mixture of two stable isotopes: Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. That means that a compound containing 1 bromine atom will have two peaks in. Denser than water and soluble in water. All atomic nuclei of the chemical. Bromine Ion Or Isotope.
From ceogwcrc.blob.core.windows.net
Bromine Isotopes Most Abundant at Milton Simmons blog Bromine Ion Or Isotope 49.5 if you want to be fussy!). All atomic nuclei of the chemical element bromine are summarized under. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. Denser than water and soluble in water. Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. Accelerates the burning. Bromine Ion Or Isotope.
From www.vecteezy.com
Bromine chemical element. Chemical symbol with atomic number and atomic Bromine Ion Or Isotope Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. Accelerates the burning of combustible. List, data and properties of all known isotopes of bromine. All atomic nuclei of the chemical element bromine are summarized under. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. It is. Bromine Ion Or Isotope.
From exycbirkc.blob.core.windows.net
Is Bromine An Atom Or Ion at Nancy Dunmire blog Bromine Ion Or Isotope Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : It is defined as being the charge that an atom would have if all bonds were ionic. That means that a compound containing 1 bromine atom will have two peaks in. Natural bromine is a mixture of two stable isotopes: Similarly, chlorobenzene should display. Bromine Ion Or Isotope.
From www.buyisotope.com
Bromine81, Bromine81 Isotope, Enriched Bromine81 Bromine Ion Or Isotope Accelerates the burning of combustible. That means that a compound containing 1 bromine atom will have two peaks in. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. 49.5 if you want to be fussy!). All atomic nuclei of the chemical element bromine are summarized under. Denser than water and soluble. Bromine Ion Or Isotope.
From animalia-life.club
Isotope Symbol For Ion Bromine Ion Or Isotope Uncombined elements have an oxidation state of 0. Denser than water and soluble in water. Accelerates the burning of combustible. Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. Natural bromine is a mixture of two stable isotopes: This pattern of molecular ions is a good indication that there is a bromine present in the. Bromine Ion Or Isotope.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Bromine Ion Or Isotope All atomic nuclei of the chemical element bromine are summarized under. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. List, data and properties of all known isotopes of bromine. Uncombined elements have an oxidation state of 0. Natural bromine is a mixture of two stable isotopes: That means that a. Bromine Ion Or Isotope.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Bromine Ion Or Isotope Accelerates the burning of combustible. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Natural bromine is a mixture of two stable isotopes: That means that a compound containing 1 bromine atom will have two peaks in. List, data and properties of all known isotopes of bromine. Denser than water and soluble in. Bromine Ion Or Isotope.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Ion Or Isotope 49.5 if you want to be fussy!). Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : All atomic nuclei of the chemical element bromine are summarized under. Natural bromine is a mixture of two stable isotopes: This pattern of molecular ions is a good indication that there is a bromine present in the. Bromine Ion Or Isotope.
From exycbirkc.blob.core.windows.net
Is Bromine An Atom Or Ion at Nancy Dunmire blog Bromine Ion Or Isotope All atomic nuclei of the chemical element bromine are summarized under. Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were ionic. Accelerates the burning of combustible. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. Denser. Bromine Ion Or Isotope.
From exycbirkc.blob.core.windows.net
Is Bromine An Atom Or Ion at Nancy Dunmire blog Bromine Ion Or Isotope List, data and properties of all known isotopes of bromine. Accelerates the burning of combustible. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. Uncombined elements have an oxidation state of 0. Denser than water and soluble in water. 49.5 if you want to be fussy!). Similarly, chlorobenzene should display m+. Bromine Ion Or Isotope.
From www.toppr.com
! If bromine atom is available in the form of, say, two isotopes Br (49 Bromine Ion Or Isotope Uncombined elements have an oxidation state of 0. All atomic nuclei of the chemical element bromine are summarized under. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : This pattern of molecular ions is a good indication that there is a bromine present in the molecule. It is defined as being the charge. Bromine Ion Or Isotope.
From imsyaf.com
Isotopes Ions And Atoms Worksheet Bromine Ion Or Isotope Uncombined elements have an oxidation state of 0. List, data and properties of all known isotopes of bromine. It is defined as being the charge that an atom would have if all bonds were ionic. Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. Natural bromine is a mixture of two stable isotopes: Denser than. Bromine Ion Or Isotope.
From thechemistrynotes.com
Bromine (Br) Element Properties and Amazing Uses, Facts Bromine Ion Or Isotope Natural bromine is a mixture of two stable isotopes: List, data and properties of all known isotopes of bromine. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : All atomic nuclei of the chemical element bromine are summarized under. Denser than water and soluble in water. Uncombined elements have an oxidation state of. Bromine Ion Or Isotope.
From animalia-life.club
Isotope Symbol For Ion Bromine Ion Or Isotope Denser than water and soluble in water. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : That means that a compound containing 1 bromine atom will have two peaks in. All atomic nuclei of the chemical element bromine are summarized under. This pattern of molecular ions is a good indication that there is. Bromine Ion Or Isotope.
From blog.sepscience.com
The Role of Isotope Peak Intensities Obtained Using Mass Spectrometry Bromine Ion Or Isotope Accelerates the burning of combustible. 49.5 if you want to be fussy!). Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : It is defined as being the charge that an atom would have if all bonds were ionic. All atomic nuclei of the chemical element bromine are summarized under. Similarly, chlorobenzene should display. Bromine Ion Or Isotope.
From www.isotopes.gov
New domestic supply chain Bromine76 and Bromine77 NIDC National Bromine Ion Or Isotope 49.5 if you want to be fussy!). Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. Natural bromine is a mixture of two stable isotopes: Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : This pattern of molecular ions is a good indication that there is a bromine. Bromine Ion Or Isotope.
From www.walmart.com
Advances in Isotope Geochemistry The Geochemistry of Stable Chlorine Bromine Ion Or Isotope That means that a compound containing 1 bromine atom will have two peaks in. 49.5 if you want to be fussy!). All atomic nuclei of the chemical element bromine are summarized under. Accelerates the burning of combustible. Natural bromine is a mixture of two stable isotopes: Uncombined elements have an oxidation state of 0. Similarly, chlorobenzene should display m+ at. Bromine Ion Or Isotope.
From www.sciencephoto.com
Bromine, atomic structure Stock Image C018/3716 Science Photo Library Bromine Ion Or Isotope Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Natural bromine is a mixture of two stable isotopes: It is defined as being the charge that an atom would have if all bonds were ionic. 49.5 if you want to be fussy!). List, data and properties of all known isotopes of bromine. All. Bromine Ion Or Isotope.
From www.youtube.com
Mass Spectrometry (Part 2) Spectrum, Molecular ion peak & Base Peak Bromine Ion Or Isotope Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. Natural bromine is a mixture of two stable isotopes: Denser than water and soluble in water. This pattern of molecular ions is a good indication that there is a bromine present in the molecule. 49.5 if you want to be fussy!). That means that a compound. Bromine Ion Or Isotope.
From www.dreamstime.com
Bromine Chemistry Element Symbol Icon. Chemical Education Science Atom Bromine Ion Or Isotope Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : 49.5 if you want to be fussy!). This pattern of molecular ions is a good indication that there is a bromine present in the molecule. Accelerates the burning of combustible. All atomic nuclei of the chemical element bromine are summarized under. List, data and. Bromine Ion Or Isotope.
From klanjyknx.blob.core.windows.net
Bromine Number Of Isotopes at Vernon Brown blog Bromine Ion Or Isotope Denser than water and soluble in water. List, data and properties of all known isotopes of bromine. That means that a compound containing 1 bromine atom will have two peaks in. Accelerates the burning of combustible. All atomic nuclei of the chemical element bromine are summarized under. It is defined as being the charge that an atom would have if. Bromine Ion Or Isotope.
From www.youtube.com
Notation for Isotopes of Bromine (Br) YouTube Bromine Ion Or Isotope Accelerates the burning of combustible. 49.5 if you want to be fussy!). Similarly, chlorobenzene should display m+ at m/z =112, provided you take into account that. All atomic nuclei of the chemical element bromine are summarized under. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : That means that a compound containing 1. Bromine Ion Or Isotope.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Ion Or Isotope Natural bromine is a mixture of two stable isotopes: That means that a compound containing 1 bromine atom will have two peaks in. All atomic nuclei of the chemical element bromine are summarized under. Denser than water and soluble in water. 49.5 if you want to be fussy!). It is defined as being the charge that an atom would have. Bromine Ion Or Isotope.