Medical Device Requirements Management Software . jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. streamline every stage of the development lifecycle with a flexible solution built for sxmd. Enable agile & digital engineering. Document review, versioning and archiving. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. #1 alm software for medical devices development.
from www.greenlight.guru
— the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Document review, versioning and archiving. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Enable agile & digital engineering. #1 alm software for medical devices development. streamline every stage of the development lifecycle with a flexible solution built for sxmd.
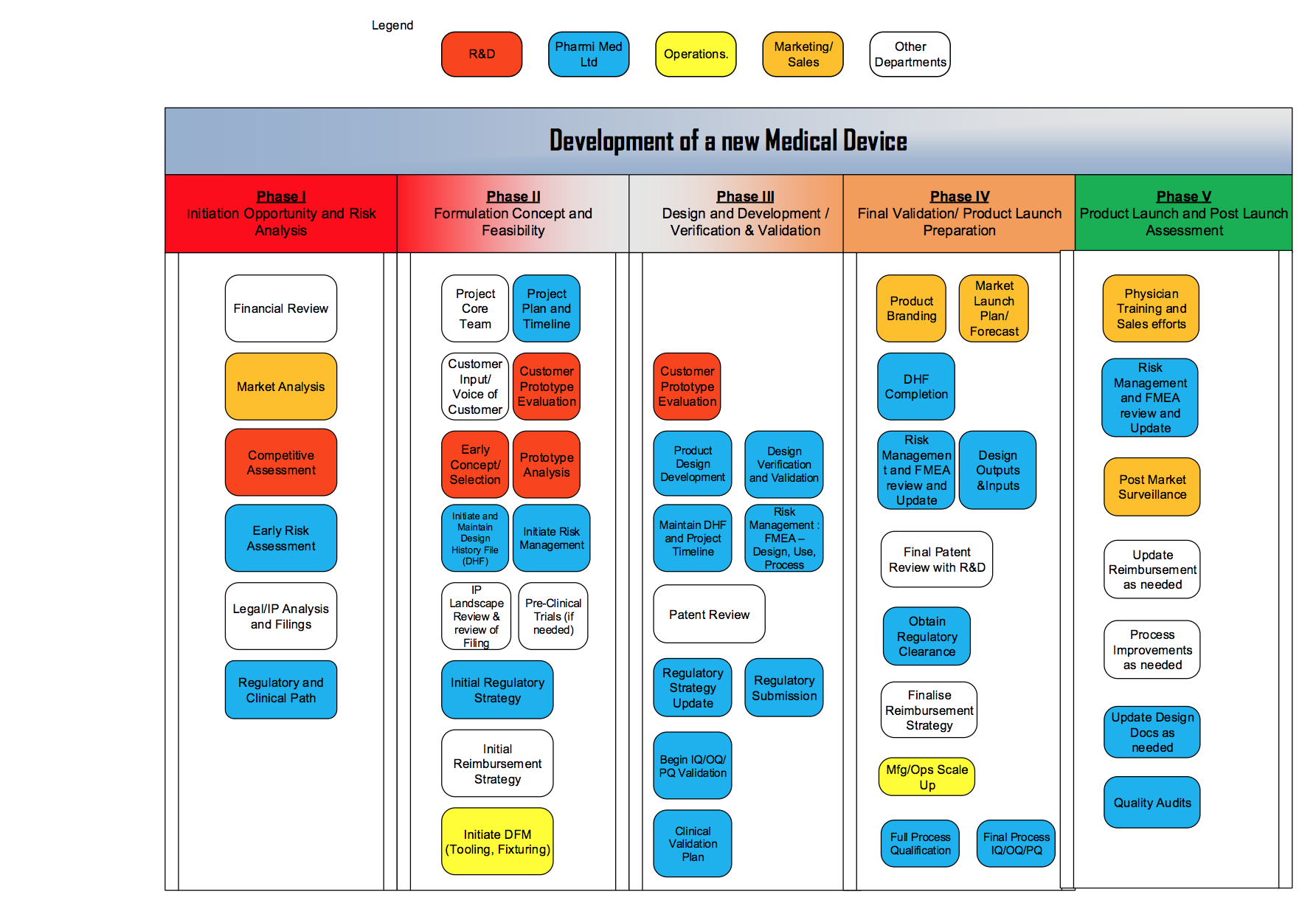
Understanding the 5 Phases of Medical Device Development
Medical Device Requirements Management Software streamline every stage of the development lifecycle with a flexible solution built for sxmd. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. streamline every stage of the development lifecycle with a flexible solution built for sxmd. Document review, versioning and archiving. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Enable agile & digital engineering. #1 alm software for medical devices development.
From www.tuleap.org
IEC 62304 compliance What are the requirements for Medical Device Medical Device Requirements Management Software jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. #1 alm software for medical devices development. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Enable agile & digital engineering. streamline every stage of the development lifecycle with. Medical Device Requirements Management Software.
From www.malvatronics.co.uk
Meeting Regulatory Requirements in Medical Device Software Development Medical Device Requirements Management Software Enable agile & digital engineering. #1 alm software for medical devices development. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. streamline every stage of the development lifecycle with a flexible solution built for sxmd. Document review, versioning and archiving. — the iec 62304 standard includes. Medical Device Requirements Management Software.
From www.qualitymeddev.com
IEC 62304 Medical Device Software Overview of the Main Requirements Medical Device Requirements Management Software Enable agile & digital engineering. #1 alm software for medical devices development. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Document review, versioning and archiving. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. streamline every stage. Medical Device Requirements Management Software.
From medicaldevicehq.com
Online + Live Virtual Requirements Engineering Course Medical Device HQ Medical Device Requirements Management Software streamline every stage of the development lifecycle with a flexible solution built for sxmd. Document review, versioning and archiving. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Enable agile. Medical Device Requirements Management Software.
From www.qualio.com
The complete guide to SaMD (software as a medical device) Medical Device Requirements Management Software Document review, versioning and archiving. #1 alm software for medical devices development. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. streamline every stage of the development lifecycle with a flexible solution built for sxmd. Enable agile & digital engineering. jama connect® for medical device & life sciences. Medical Device Requirements Management Software.
From www.modernrequirements.com
Facilitate the Medical Device Design Controls Modern Requirements Medical Device Requirements Management Software jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. #1 alm software for medical devices development. Enable agile & digital engineering. streamline every stage of the development lifecycle with. Medical Device Requirements Management Software.
From www.orielstat.com
FDA and EU Risk Requirements for Medical Device Software & SaMD Oriel Medical Device Requirements Management Software #1 alm software for medical devices development. streamline every stage of the development lifecycle with a flexible solution built for sxmd. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams. Medical Device Requirements Management Software.
From www.pcbdirectory.com
Understanding the PCB Requirements for Medical Applications PCB Directory Medical Device Requirements Management Software Enable agile & digital engineering. #1 alm software for medical devices development. streamline every stage of the development lifecycle with a flexible solution built for sxmd. Document review, versioning and archiving. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. — the iec 62304 standard includes. Medical Device Requirements Management Software.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key Medical Device Requirements Management Software streamline every stage of the development lifecycle with a flexible solution built for sxmd. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Enable agile & digital engineering. Document review, versioning and archiving. — the iec 62304 standard includes requirements for managing the life cycle of medical. Medical Device Requirements Management Software.
From medicaldevicehq.com
An overview of the IEC 62304 standard and software safety Medical Device Requirements Management Software jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Enable agile & digital engineering. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. #1 alm software for medical devices development. Document review, versioning and archiving. streamline every stage. Medical Device Requirements Management Software.
From medicaldeviceacademy.com
Define medical device software verification and validation (V&V Medical Device Requirements Management Software #1 alm software for medical devices development. Document review, versioning and archiving. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Enable agile & digital engineering. streamline every stage of the development lifecycle with a flexible solution built for sxmd. — the iec 62304 standard includes. Medical Device Requirements Management Software.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Requirements Management Software jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Document review, versioning and archiving. streamline every stage of the development lifecycle with a flexible solution built for sxmd. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Enable agile. Medical Device Requirements Management Software.
From tateeda.com
Medical Device Software Development The Complete Guide TATEEDA GLOBAL Medical Device Requirements Management Software jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Enable agile & digital engineering. streamline every stage of the development lifecycle with a flexible solution built for sxmd. #1 alm software for medical devices development. — the iec 62304 standard includes requirements for managing the life. Medical Device Requirements Management Software.
From iziel.com
QMS Documentation for Medical Devices ISO 13485 Certification IZiel Medical Device Requirements Management Software — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. streamline every stage of the development lifecycle with a flexible solution built for sxmd. Enable agile & digital engineering. #1 alm software for medical devices development. jama connect® for medical device & life sciences development is a single powerful. Medical Device Requirements Management Software.
From www.selecthub.com
Medical Practice Management Software Features & Requirements Medical Device Requirements Management Software Document review, versioning and archiving. streamline every stage of the development lifecycle with a flexible solution built for sxmd. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. #1 alm software for medical devices development. jama connect® for medical device & life sciences development is a single powerful. Medical Device Requirements Management Software.
From digitalhealth.folio3.com
FDA Software Guide FDA software as a medical device Medical Device Requirements Management Software Enable agile & digital engineering. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Document review, versioning and archiving. streamline every stage of the development lifecycle with a flexible solution built for sxmd. #1 alm software for medical devices development. jama connect® for medical device & life sciences. Medical Device Requirements Management Software.
From financesonline.com
Best Medical Practice Management Software in 2024 Medical Device Requirements Management Software Document review, versioning and archiving. #1 alm software for medical devices development. streamline every stage of the development lifecycle with a flexible solution built for sxmd. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. — the iec 62304 standard includes requirements for managing the life. Medical Device Requirements Management Software.
From kantify.ai
Kantify Improving Human Health through Artificial Intelligence Medical Device Requirements Management Software streamline every stage of the development lifecycle with a flexible solution built for sxmd. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Enable agile & digital engineering. Document review, versioning and archiving. jama connect® for medical device & life sciences development is a single powerful platform for medical. Medical Device Requirements Management Software.
From operonstrategist.com
Software as a Medical Device (SaMD) IEC 62304 Certification Medical Device Requirements Management Software Document review, versioning and archiving. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Enable agile & digital engineering. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. #1 alm software for medical devices development. streamline every stage. Medical Device Requirements Management Software.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Requirements Management Software Enable agile & digital engineering. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. #1 alm software for medical devices development. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Document review, versioning and archiving. streamline every stage. Medical Device Requirements Management Software.
From simbex.com
How to Define Product Requirements for Medical Devices Medical Device Requirements Management Software streamline every stage of the development lifecycle with a flexible solution built for sxmd. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Enable agile & digital engineering. Document review,. Medical Device Requirements Management Software.
From www.cognidox.com
4 ways to build a medical device quality management system Medical Device Requirements Management Software jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Enable agile & digital engineering. streamline every stage of the development lifecycle with a flexible solution built for sxmd. Document review,. Medical Device Requirements Management Software.
From jelvix.com
Why is SaMD? Examples of Software as a Medical Device. Medical Device Requirements Management Software streamline every stage of the development lifecycle with a flexible solution built for sxmd. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Document review, versioning and archiving. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. #1. Medical Device Requirements Management Software.
From www.mgtechsoft.com
Software for medical devices including SaMD & SiMD MicroGenesis Medical Device Requirements Management Software Enable agile & digital engineering. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Document review, versioning and archiving. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. #1 alm software for medical devices development. streamline every stage. Medical Device Requirements Management Software.
From instantgmp.com
All in One Medical Device Quality Software InstantGMP Medical Device Requirements Management Software Enable agile & digital engineering. streamline every stage of the development lifecycle with a flexible solution built for sxmd. #1 alm software for medical devices development. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. — the iec 62304 standard includes requirements for managing the life. Medical Device Requirements Management Software.
From www.perforce.com
Condensed Guide to Medical Device Requirements Management Perforce Medical Device Requirements Management Software Document review, versioning and archiving. Enable agile & digital engineering. #1 alm software for medical devices development. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. streamline every stage. Medical Device Requirements Management Software.
From biosistemika.com
IVD medical device software development regulations Medical Device Requirements Management Software jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. streamline every stage of the development lifecycle with a flexible solution built for sxmd. Enable agile & digital engineering. #1 alm software for medical devices development. — the iec 62304 standard includes requirements for managing the life. Medical Device Requirements Management Software.
From www.regdesk.co
TGA FAQ on Softwarebased Medical Devices RegDesk Medical Device Requirements Management Software streamline every stage of the development lifecycle with a flexible solution built for sxmd. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Document review, versioning and archiving. #1 alm software for medical devices development. — the iec 62304 standard includes requirements for managing the life. Medical Device Requirements Management Software.
From rewisoft.com
Guide To Medical Device Software Development — RewiSoft Medical Device Requirements Management Software Document review, versioning and archiving. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. #1 alm software for medical devices development. streamline every stage of the development lifecycle with a flexible solution built for sxmd. Enable agile & digital engineering. jama connect® for medical device & life sciences. Medical Device Requirements Management Software.
From www.ics.com
What You Need to Know About Developing Software as a Medical Device ICS Medical Device Requirements Management Software streamline every stage of the development lifecycle with a flexible solution built for sxmd. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Enable agile & digital engineering. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Document review,. Medical Device Requirements Management Software.
From www.selecthub.com
Medical Software Features And Requirements For 2024 Medical Device Requirements Management Software #1 alm software for medical devices development. Document review, versioning and archiving. — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Enable agile & digital engineering. streamline every stage of the development lifecycle with a flexible solution built for sxmd. jama connect® for medical device & life sciences. Medical Device Requirements Management Software.
From www.regdesk.co
MDCG Visual Guide to Medical Device Software RegDesk Medical Device Requirements Management Software streamline every stage of the development lifecycle with a flexible solution built for sxmd. Document review, versioning and archiving. Enable agile & digital engineering. #1 alm software for medical devices development. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. — the iec 62304 standard includes. Medical Device Requirements Management Software.
From www.pinterest.at
ISO 13485 Basics and How to Get Started (QMS for Medical Devices Medical Device Requirements Management Software Document review, versioning and archiving. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. #1 alm software for medical devices development. Enable agile & digital engineering. streamline every stage of the development lifecycle with a flexible solution built for sxmd. — the iec 62304 standard includes. Medical Device Requirements Management Software.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Requirements Management Software — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Enable agile & digital engineering. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. Document review, versioning and archiving. #1 alm software for medical devices development. streamline every stage. Medical Device Requirements Management Software.
From www.medtextpert.com
8 Facts Every Medical Software Developer Should Know About the MDR Medical Device Requirements Management Software — the iec 62304 standard includes requirements for managing the life cycle of medical device software, from. Enable agile & digital engineering. jama connect® for medical device & life sciences development is a single powerful platform for medical device teams to. #1 alm software for medical devices development. streamline every stage of the development lifecycle with. Medical Device Requirements Management Software.