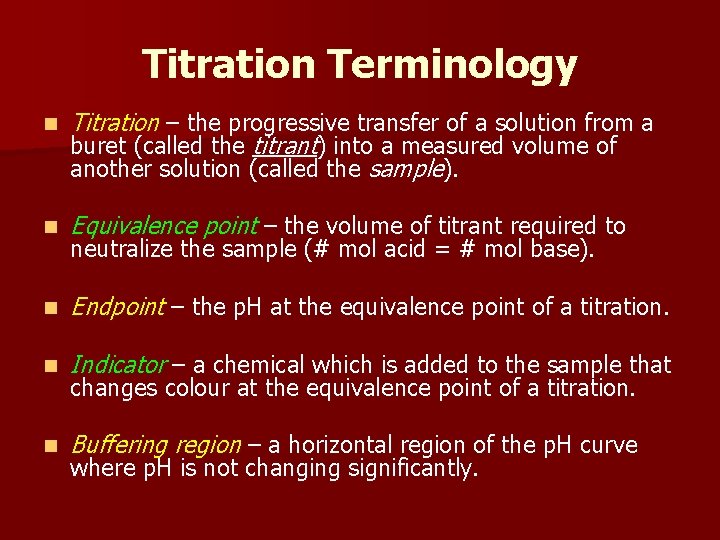
Titration Changes Meaning . titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. — titration is the process in which one solution is added to another solution such that it. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be.
from slidetodoc.com
a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. — titration is the process in which one solution is added to another solution such that it. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be.
Acid Base Titrations Changes in AcidBase Reaction Systems
Titration Changes Meaning — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — titration is the process in which one solution is added to another solution such that it. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Changes Meaning titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to. Titration Changes Meaning.
From mungfali.com
Acid Base Titration Calculation Titration Changes Meaning — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — titration is the process in which one solution is added to another solution. Titration Changes Meaning.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Changes Meaning — titration is the process in which one solution is added to another solution such that it. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. — titration, process of chemical analysis in which the quantity of some constituent of. Titration Changes Meaning.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Changes Meaning — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration Changes Meaning.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Changes Meaning titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. — titration is the process in which one solution is added to another solution such that it. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a. Titration Changes Meaning.
From printablevozljevrc.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Changes Meaning — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. — titration is the process in which one solution is added to another solution such that. Titration Changes Meaning.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Changes Meaning — titration is the process in which one solution is added to another solution such that it. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Titration Changes Meaning.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration Changes Meaning — titration is the process in which one solution is added to another solution such that it. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Titration Changes Meaning.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Changes Meaning titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is the process of determining the quantity of one substance by adding measured amounts. Titration Changes Meaning.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Changes Meaning titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,.. Titration Changes Meaning.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Changes Meaning titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a titration is the process of determining the quantity of one. Titration Changes Meaning.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Changes Meaning a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. — titration, process of chemical analysis in which the quantity of some. Titration Changes Meaning.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Titration Changes Meaning titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. — titration is the process in which one solution is added to another solution such that it. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is. Titration Changes Meaning.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Changes Meaning titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method. Titration Changes Meaning.
From dxobsfqym.blob.core.windows.net
Titration Indicators Meaning at Angela Armstrong blog Titration Changes Meaning titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. — titration is the process in which one solution is added to another solution. Titration Changes Meaning.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Changes Meaning — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. — titration is the process in which one solution is added to another solution such that it. a titration is the process of determining the quantity of one substance by adding. Titration Changes Meaning.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Changes Meaning titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. Titration Changes Meaning.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Changes Meaning — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis. Titration Changes Meaning.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Changes Meaning — titration is the process in which one solution is added to another solution such that it. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to. Titration Changes Meaning.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Changes Meaning Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. — a titration is a volumetric technique in which a solution of one reactant (the titrant). Titration Changes Meaning.
From slideplayer.com
Titration Colour Changes ppt download Titration Changes Meaning titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Titration Changes Meaning.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Changes Meaning — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory. Titration Changes Meaning.
From resource.studiaacademy.com
Acids, Alkalis and Titrations Studia Academy Resources Titration Changes Meaning titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. — a titration is a volumetric technique in which a solution of one reactant. Titration Changes Meaning.
From www.slideserve.com
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free Titration Changes Meaning — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of. Titration Changes Meaning.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Changes Meaning titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. — titration is the process in which one solution is added to another solution such that it. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration Changes Meaning.
From www.sliderbase.com
Titration Titration Changes Meaning — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. — titration is the process in which one solution is added to another. Titration Changes Meaning.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Titration Changes Meaning Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. — titration, process of chemical analysis in which the quantity of some constituent of. Titration Changes Meaning.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Changes Meaning — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. a titration is the process of determining the quantity of one substance by. Titration Changes Meaning.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Changes Meaning a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. — a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. — titration is the process in which one solution is added to another solution such that it.. Titration Changes Meaning.
From slidetodoc.com
Acid Base Titrations Changes in AcidBase Reaction Systems Titration Changes Meaning — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. — titration is the process in which one solution is added to another solution such that it. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Changes Meaning.
From stock.adobe.com
Acid base titration experiment and phases of color change during Titration Changes Meaning — titration is the process in which one solution is added to another solution such that it. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined.. Titration Changes Meaning.
From exovodadv.blob.core.windows.net
Titration End Point Meaning at Jenny Ingram blog Titration Changes Meaning Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. — titration is the process in which one solution is added to another solution such that it.. Titration Changes Meaning.
From present5.com
Titration and AcidBase Neutralization LEARNING OBJECTIVES 11.2.2.7 Titration Changes Meaning titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. — titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. — titration is the process in which one solution is added to another solution such that. Titration Changes Meaning.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Titration Changes Meaning — titration is the process in which one solution is added to another solution such that it. a titration is the process of determining the quantity of one substance by adding measured amounts of another substance,. titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the. . Titration Changes Meaning.