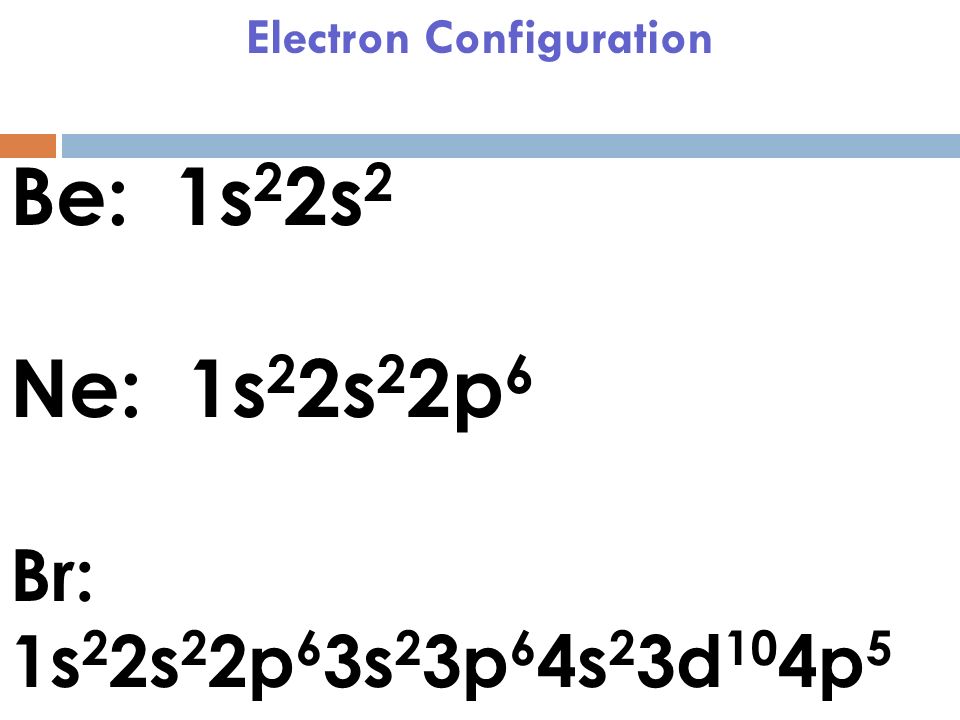
Bromine Atom Orbitals . These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. For example, bromine takes one electron and becomes isoelectronic to t kr: This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. Bromine atomic orbital and chemical bonding information. This diagram shows how the electrons in the bromine.
from periodictable.me
For example, bromine takes one electron and becomes isoelectronic to t kr: The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. Bromine atomic orbital and chemical bonding information. These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. This diagram shows how the electrons in the bromine.
Bromine Electron Configuration (Br) with Orbital Diagram
Bromine Atom Orbitals These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. For example, bromine takes one electron and becomes isoelectronic to t kr: Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. This diagram shows how the electrons in the bromine. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. Bromine atomic orbital and chemical bonding information. This can be shortened to #[ar] 4s^2 3d^10 4p^5#.
From www.numerade.com
SOLVEDThe bromine atom possesses 35 electrons. It contains 6 electrons in 2 p orbital, 6 Bromine Atom Orbitals For example, bromine takes one electron and becomes isoelectronic to t kr: These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. Bromine atomic orbital and chemical bonding information. This diagram shows how the electrons in the bromine. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s. Bromine Atom Orbitals.
From www.youtube.com
Copy of Electron configuration, Orbital notation and Quantum Numbers for Bromine YouTube Bromine Atom Orbitals For example, bromine takes one electron and becomes isoelectronic to t kr: Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. Instead, the bonding in ethene is described by a model involving the participation of a different kind of. Bromine Atom Orbitals.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Orbitals The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. Bromine atomic orbital and chemical bonding information. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. This diagram shows how the electrons in the. Bromine Atom Orbitals.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron configuration of an atom of Bromine Atom Orbitals For example, bromine takes one electron and becomes isoelectronic to t kr: Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. Bromine atomic orbital and chemical bonding information. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6. Bromine Atom Orbitals.
From app.emaze.com
Bromine ) on emaze Bromine Atom Orbitals The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. This diagram shows how the electrons in the bromine. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. These new combinations are called hybrid. Bromine Atom Orbitals.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Bromine Atom Orbitals Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. This diagram shows how the electrons in the bromine. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. For. Bromine Atom Orbitals.
From www.breakingatom.com
Bromine (Br) Atomic Number 35 Bromine Atom Orbitals This diagram shows how the electrons in the bromine. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. Bromine atomic orbital and chemical bonding information. This can be shortened to #[ar] 4s^2 3d^10. Bromine Atom Orbitals.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration of nucleus, particle Bromine Atom Orbitals For example, bromine takes one electron and becomes isoelectronic to t kr: Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. Bromine atomic orbital and chemical bonding information. The bromine orbital diagram is a graphical representation of the electron. Bromine Atom Orbitals.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Atom Orbitals For example, bromine takes one electron and becomes isoelectronic to t kr: This diagram shows how the electrons in the bromine. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. Br([ar] 4s 2 3d 10 4p 5). Bromine Atom Orbitals.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Atom Orbitals The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. For example, bromine takes one electron and becomes isoelectronic to t kr: The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#.. Bromine Atom Orbitals.
From favpng.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Model, PNG, 558x600px Bromine Atom Orbitals For example, bromine takes one electron and becomes isoelectronic to t kr: Bromine atomic orbital and chemical bonding information. This diagram shows how the electrons in the bromine. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. Bromine Atom Orbitals.
From schematron.org
Bromine Bohr Diagram Wiring Diagram Pictures Bromine Atom Orbitals This diagram shows how the electrons in the bromine. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. For example, bromine takes one electron and. Bromine Atom Orbitals.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Protons are represented as Bromine Atom Orbitals For example, bromine takes one electron and becomes isoelectronic to t kr: Bromine atomic orbital and chemical bonding information. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron. Bromine Atom Orbitals.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Atom Orbitals This diagram shows how the electrons in the bromine. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine atomic orbital and chemical bonding information. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. For example, bromine takes one. Bromine Atom Orbitals.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Atom Orbitals Bromine atomic orbital and chemical bonding information. For example, bromine takes one electron and becomes isoelectronic to t kr: These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Instead, the bonding in ethene is described by a model involving the participation of. Bromine Atom Orbitals.
From www.shutterstock.com
Symbol Electron Diagram Bromine Illustration Stock Vector (Royalty Free) 318671348 Bromine Atom Orbitals Bromine atomic orbital and chemical bonding information. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. For example, bromine takes one electron and becomes isoelectronic to t kr: This diagram shows. Bromine Atom Orbitals.
From quizlet.com
How to draw the orbital diagram of bromine? Quizlet Bromine Atom Orbitals The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. For example, bromine takes one electron and becomes isoelectronic to t kr: This diagram shows how the electrons in the bromine. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#.. Bromine Atom Orbitals.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Orbitals These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine atomic orbital and chemical bonding information. For example, bromine takes one electron and becomes isoelectronic to t kr: Br([ar] 4s 2 3d 10 4p 5) + e. Bromine Atom Orbitals.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Orbitals The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. For example, bromine takes one electron and becomes isoelectronic to t kr: These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#.. Bromine Atom Orbitals.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine Bromine Atom Orbitals The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. For example, bromine takes one electron and becomes isoelectronic to t kr: Instead, the bonding in ethene is described by a. Bromine Atom Orbitals.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Orbitals Bromine atomic orbital and chemical bonding information. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. This diagram shows how the electrons in the bromine. For example, bromine takes one electron and becomes isoelectronic to t kr: These new. Bromine Atom Orbitals.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron configuration, and valence orbitals Bromine Atom Orbitals These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. This diagram shows how the electrons in the bromine. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. The electron configuration. Bromine Atom Orbitals.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Bromine Atom Orbitals These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. This diagram shows how the electrons in the bromine. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. Instead, the bonding. Bromine Atom Orbitals.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Bromine (Br) YouTube Bromine Atom Orbitals Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. This diagram shows how the electrons in the bromine. For example, bromine takes one electron and becomes isoelectronic to t kr: These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. This. Bromine Atom Orbitals.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Atom Orbitals The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine atomic orbital. Bromine Atom Orbitals.
From www.peoi.org
Chapter 8 Section B Quantum Numbers for Electrons Bromine Atom Orbitals The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. For example, bromine takes one electron and becomes isoelectronic to t kr: Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. Bromine atomic orbital and chemical. Bromine Atom Orbitals.
From cms.gutow.uwosh.edu
Bromine Bromine Atom Orbitals The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. For example, bromine takes one electron and becomes isoelectronic to. Bromine Atom Orbitals.
From www.numerade.com
SOLVED a) How many electroncontaining orbitals does a bromine atom have in the ground state? b Bromine Atom Orbitals For example, bromine takes one electron and becomes isoelectronic to t kr: Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. This diagram shows how the electrons in the bromine. These new combinations. Bromine Atom Orbitals.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine Bromine Atom Orbitals Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine atomic orbital and chemical. Bromine Atom Orbitals.
From pnghut.com
Electron Configuration Shell Atom Bromine Atomic Orbital Selfintroduction Transparent PNG Bromine Atom Orbitals Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. Br([ar] 4s 2 3d 10 4p 5) +. Bromine Atom Orbitals.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron configuration, chemical data, and Bromine Atom Orbitals This diagram shows how the electrons in the bromine. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. The bromine orbital diagram is a graphical representation of the electron configuration. Bromine Atom Orbitals.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image & Art Alamy Bromine Atom Orbitals Bromine atomic orbital and chemical bonding information. The bromine orbital diagram is a graphical representation of the electron configuration of the bromine atom. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s. Bromine Atom Orbitals.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine Atom Orbitals This diagram shows how the electrons in the bromine. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. These new combinations are called hybrid atomic orbitals because they are produced by combining (hybridizing) two or more. Instead, the bonding in ethene is described by a model. Bromine Atom Orbitals.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Table of the Elements Bromine Atom Orbitals For example, bromine takes one electron and becomes isoelectronic to t kr: The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Instead, the bonding in ethene is described by a model involving the participation of a different kind of hybrid orbital. These new combinations are called. Bromine Atom Orbitals.
From quizlet.com
How to draw the orbital diagram of bromine? Quizlet Bromine Atom Orbitals Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic with kr ([ar] 4s 2 3d 10 4p 6)] orbital. For example, bromine takes one electron and becomes isoelectronic to t kr: This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6. Bromine Atom Orbitals.