Properties Of Magnesia . Its boiling point is 1090ºc or 1994ºf. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. But when water bodies are accounted. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. Among all the structural metals, magnesium is the lightest. Magnesium has a silvery white appearance. Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. In hot water, the reaction is more vigorous.
from agutsygirl.com
Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. But when water bodies are accounted. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. In hot water, the reaction is more vigorous. Among all the structural metals, magnesium is the lightest. Magnesium has a silvery white appearance. Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. Its boiling point is 1090ºc or 1994ºf.
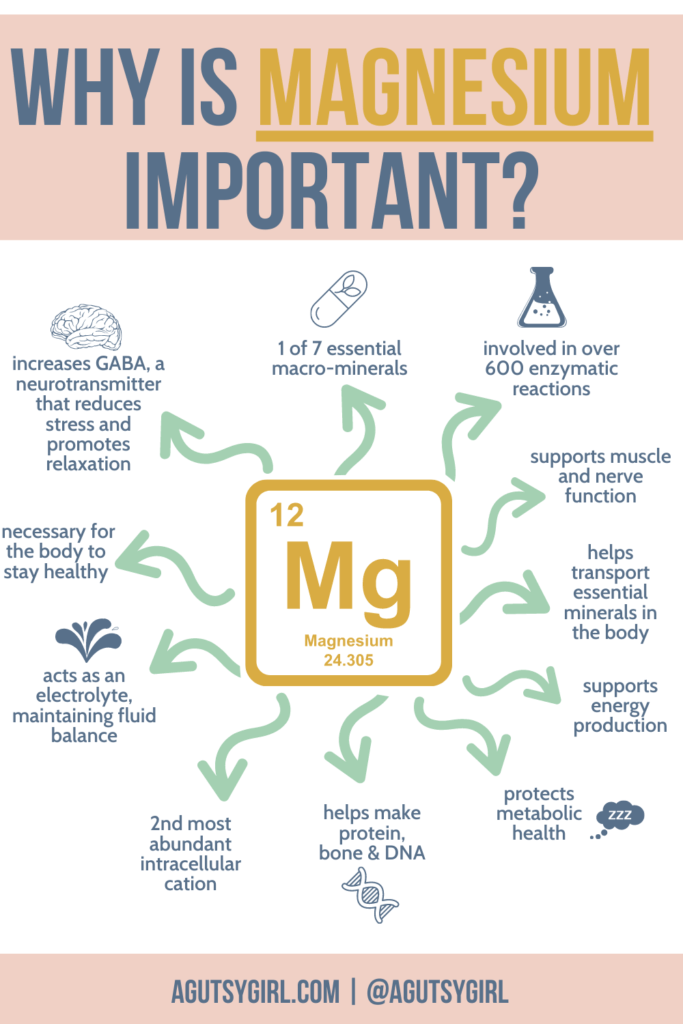
Types of Magnesium {Your Master Guide} A Gutsy Girl®
Properties Of Magnesia Among all the structural metals, magnesium is the lightest. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Magnesium has a silvery white appearance. In hot water, the reaction is more vigorous. Its boiling point is 1090ºc or 1994ºf. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. Among all the structural metals, magnesium is the lightest. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. But when water bodies are accounted. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Properties Of Magnesia Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. Its boiling point is 1090ºc or 1994ºf. In hot water, the reaction is more vigorous. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most. Properties Of Magnesia.
From crystal-information.com
Magnesium Properties and Meaning + Photos Crystal Information Properties Of Magnesia Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. Magnesium has a silvery white appearance. But when water bodies are accounted. Among all the structural metals, magnesium is the lightest. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali. Properties Of Magnesia.
From www.examples.com
Magnesium (Mg) Definition, Preparation, Properties, Uses, Compounds Properties Of Magnesia Magnesium has a silvery white appearance. But when water bodies are accounted. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Its boiling point is 1090ºc or 1994ºf. There are over 60 different. Properties Of Magnesia.
From www.academia.edu
(PDF) Mechanical properties of magnesium casting alloys Tomasz Tański Properties Of Magnesia Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Among all the structural metals, magnesium is the lightest. Its boiling point is 1090ºc or 1994ºf. There are over 60 different minerals known to. Properties Of Magnesia.
From pediaa.com
Difference Between Magnesium and Magnesium Oxide Definition Properties Of Magnesia Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Among all the structural metals, magnesium is the lightest. But when water bodies are accounted. Its boiling point is 1090ºc or 1994ºf. There are over 60 different minerals. Properties Of Magnesia.
From www.nuclear-power.com
What is Magnesium Properties of Magnesium Element Symbol Mg Properties Of Magnesia It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. But when water bodies are accounted. In hot water, the reaction is more vigorous. Magnesium has a. Properties Of Magnesia.
From www.allthescience.org
What are the Properties of Magnesium? (with pictures) Properties Of Magnesia Its boiling point is 1090ºc or 1994ºf. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. But when water bodies are accounted. Magnesium is the lightest of all metal elements and is primarily used in structural alloys. Properties Of Magnesia.
From www.researchgate.net
Properties of porous magnesia composites Download Table Properties Of Magnesia Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Its boiling point is 1090ºc or 1994ºf. But when water bodies are accounted. It reacts with water at room temperature, albeit slowly, to form. Properties Of Magnesia.
From blog.thepipingmart.com
Magnesium Properties A Complete Guide Properties Of Magnesia In hot water, the reaction is more vigorous. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. Magnesium has a silvery white. Properties Of Magnesia.
From www.youtube.com
Magnesium(Chemical Properties) YouTube Properties Of Magnesia Among all the structural metals, magnesium is the lightest. In hot water, the reaction is more vigorous. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to. Properties Of Magnesia.
From blog.thepipingmart.com
5 Physical Properties of Magnesium Properties Of Magnesia In hot water, the reaction is more vigorous. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. But when water bodies are accounted. Magnesium has a silvery white appearance. Among all the structural metals, magnesium is the lightest. Its boiling point is 1090ºc or 1994ºf. Magnesium is used in products that benefit from. Properties Of Magnesia.
From studiousguy.com
Magnesium (Mg) Properties & Uses StudiousGuy Properties Of Magnesia In hot water, the reaction is more vigorous. But when water bodies are accounted. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. There are over 60 different minerals known to have a. Properties Of Magnesia.
From www.researchgate.net
Physical properties of magnesium hybrid composites Download Properties Of Magnesia Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Magnesium has a silvery white appearance. Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. There are over 60 different minerals known to have a. Properties Of Magnesia.
From blog.thepipingmart.com
Magnesium Properties and Uses Properties Of Magnesia In hot water, the reaction is more vigorous. But when water bodies are accounted. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. There are over 60 different minerals known to have a. Properties Of Magnesia.
From irchemineral.com
Magnesium sulfate properties IRCHEM.co Properties Of Magnesia Its boiling point is 1090ºc or 1994ºf. Magnesium has a silvery white appearance. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. It reacts with water. Properties Of Magnesia.
From www.britannica.com
magnesium Description, Properties, & Compounds Britannica Properties Of Magnesia Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Among all the structural metals, magnesium is the lightest. In hot water, the reaction is more. Properties Of Magnesia.
From studylib.net
The Properties of Magnesia Portland Cement Properties Of Magnesia There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. But when water bodies are accounted. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. In hot water, the reaction is more. Properties Of Magnesia.
From www.dreamstime.com
Element of Magnesium stock illustration. Illustration of clip 89525283 Properties Of Magnesia There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Its boiling point is 1090ºc or 1994ºf. Magnesium is quite reactive, especially when powdered, though less so. Properties Of Magnesia.
From www.researchgate.net
properties and chemical compositions of the selected magnesia sources Properties Of Magnesia Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. In hot water, the reaction is more vigorous. Magnesium has a silvery white appearance. Magnesium is used in products that benefit from being lightweight, such as car seats,. Properties Of Magnesia.
From agutsygirl.com
Types of Magnesium {Your Master Guide} A Gutsy Girl® Properties Of Magnesia Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. In hot water, the reaction is more vigorous. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Magnesium is quite reactive, especially when powdered, though less so than the. Properties Of Magnesia.
From www.slideshare.net
Magnesium Properties Of Magnesia Magnesium has a silvery white appearance. In hot water, the reaction is more vigorous. Among all the structural metals, magnesium is the lightest. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most. Properties Of Magnesia.
From scienceinfo.com
Magnesium (Mg) Element Properties, Uses, Interesting Facts Properties Of Magnesia Among all the structural metals, magnesium is the lightest. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. Magnesium has a silvery white appearance. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. Magnesium is. Properties Of Magnesia.
From periodictableguide.com
Magnesium (Mg) Periodic Table (Element Information & More) Properties Of Magnesia Among all the structural metals, magnesium is the lightest. But when water bodies are accounted. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. In hot water, the reaction is more vigorous. Magnesium is used in products. Properties Of Magnesia.
From www.slideserve.com
PPT Metals and the Discoveries PowerPoint Presentation, free download Properties Of Magnesia It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Its boiling point is 1090ºc or 1994ºf. Magnesium has a silvery white appearance. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. Magnesium is the lightest. Properties Of Magnesia.
From chempedia.info
Physical and Chemical Properties of Magnesium Oxide Big Chemical Properties Of Magnesia In hot water, the reaction is more vigorous. Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Its boiling point is 1090ºc or 1994ºf. But when water bodies are. Properties Of Magnesia.
From www.slideserve.com
PPT Magnesium PowerPoint Presentation, free download ID2697972 Properties Of Magnesia Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas.. Properties Of Magnesia.
From www.haikudeck.com
Project by Russell Holland Properties Of Magnesia Among all the structural metals, magnesium is the lightest. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the. Properties Of Magnesia.
From crystal-information.com
Magnesium Properties and Meaning + Photos Crystal Information Properties Of Magnesia Magnesium has a silvery white appearance. Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. Among all the structural metals, magnesium is the lightest. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Magnesium. Properties Of Magnesia.
From www.slideshare.net
03 magnesium and magnesium alloys Properties Of Magnesia There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Its boiling point is 1090ºc or 1994ºf. But when water bodies are accounted. Magnesium has a silvery. Properties Of Magnesia.
From www.alamy.com
Symbol and electron diagram for Magnesium illustration Stock Vector Properties Of Magnesia Among all the structural metals, magnesium is the lightest. Its boiling point is 1090ºc or 1994ºf. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Magnesium has a silvery white appearance. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. It reacts. Properties Of Magnesia.
From www.youtube.com
Magnesium *** YouTube Properties Of Magnesia Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Its boiling point is 1090ºc or 1994ºf. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. Among. Properties Of Magnesia.
From blog.thepipingmart.com
The Chemical Properties of Magnesium Properties Of Magnesia Among all the structural metals, magnesium is the lightest. It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. There are over 60 different minerals known to have a magnesium content of 20% or greater, making it the eighth most abundant element in the earth's crust. Magnesium is the lightest of all metal elements. Properties Of Magnesia.
From www.semanticscholar.org
Table 1 from Properties of magnesium based cements Semantic Scholar Properties Of Magnesia Among all the structural metals, magnesium is the lightest. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. Magnesium is the lightest of all metal elements and is primarily used in structural alloys due to its lightweight, strength, and resistance to corrosion. Magnesium has a silvery white appearance. It reacts with water at. Properties Of Magnesia.
From www.thoughtco.com
Magnesium Characteristics, Properties, and Applications Properties Of Magnesia It reacts with water at room temperature, albeit slowly, to form magnesium hydroxide and hydrogen gas. But when water bodies are accounted. Among all the structural metals, magnesium is the lightest. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. There are over 60 different minerals known to have. Properties Of Magnesia.
From www.slideserve.com
PPT Magnesium PowerPoint Presentation, free download ID1733044 Properties Of Magnesia Its boiling point is 1090ºc or 1994ºf. Magnesium has a silvery white appearance. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. Magnesium is quite reactive, especially when powdered, though less so than the more reactive alkali metals. Among all the structural metals, magnesium is the lightest. In hot. Properties Of Magnesia.