Electrochemistry Bipolar Electrode . Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase.
from www.mdpi.com
Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply.
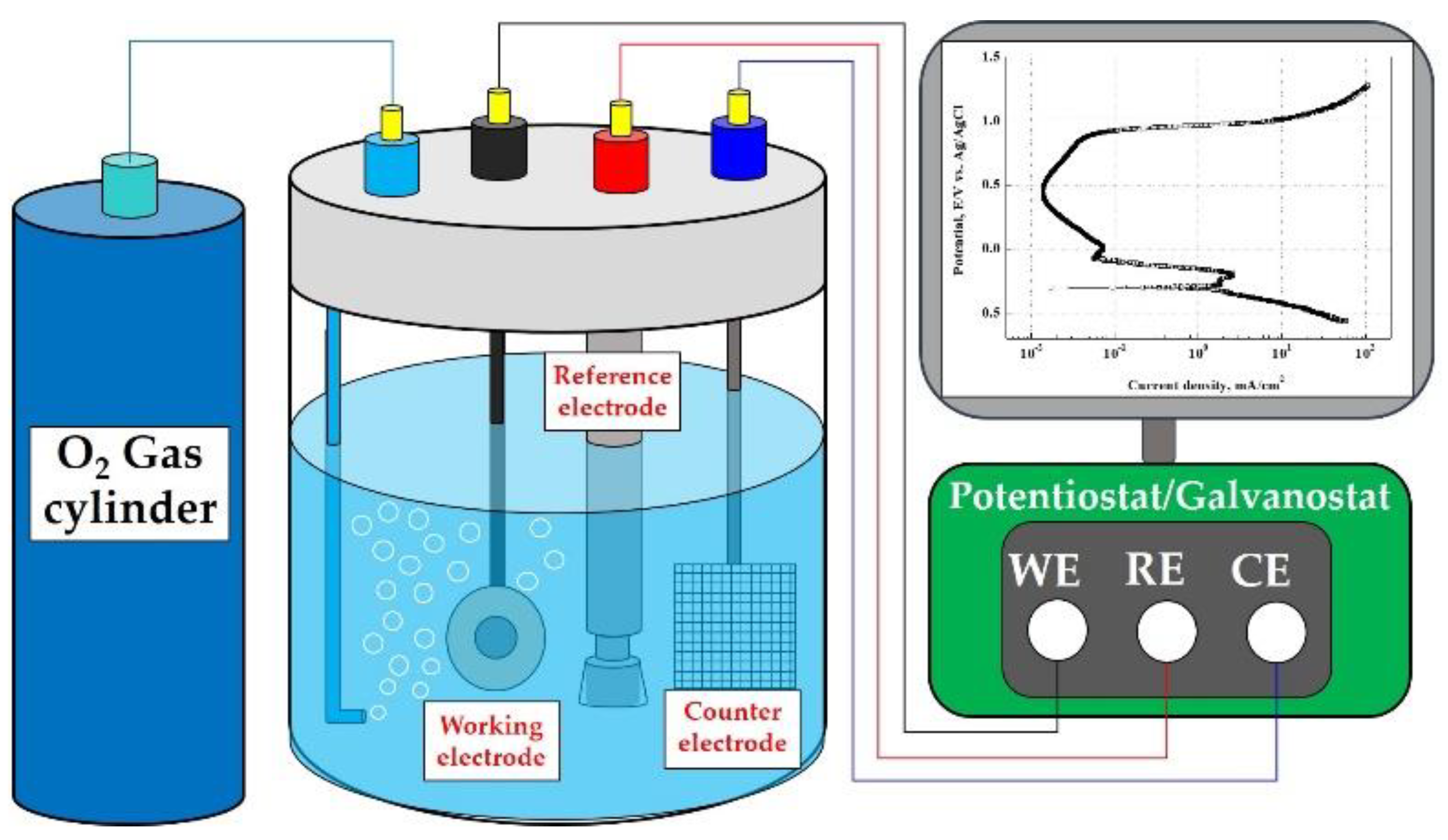
Coatings Free FullText Electrochemical Characteristics with NaCl
Electrochemistry Bipolar Electrode A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply.
From www.mdpi.com
Biosensors Free FullText Newly Developed Electrochemiluminescence Electrochemistry Bipolar Electrode Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. 1 the usual setup for bpe contains. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Design and achievement of bipolar electrode structure. a Schematic of Electrochemistry Bipolar Electrode When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. A different mode for driving electrochemical reactions has recently. Electrochemistry Bipolar Electrode.
From chemistry-europe.onlinelibrary.wiley.com
Bipolar Electrochemistry A Powerful Tool for Micro/Nano Electrochemistry Bipolar Electrode When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. A different mode for. Electrochemistry Bipolar Electrode.
From www.mdpi.com
Biosensors Free FullText Newly Developed Electrochemiluminescence Electrochemistry Bipolar Electrode A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an.. Electrochemistry Bipolar Electrode.
From www.mdpi.com
Micromachines Free FullText Electrochemistry in a Two or Three Electrochemistry Bipolar Electrode In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Schematic illustration of bipolar electrochemical setup for Electrochemistry Bipolar Electrode When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). A bipolar electrode (bpe) is an electronic conductor. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Schematic representation of the principle of bipolar electrochemistry Electrochemistry Bipolar Electrode Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Schematic illustration of the principle of bipolar electrochemistry Electrochemistry Bipolar Electrode A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Bipolar electrochemistry is when a metal at open circuit Electrochemistry Bipolar Electrode 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical. Electrochemistry Bipolar Electrode.
From www.researchgate.net
(a) Construction of the closed bipolar electrode system to physically Electrochemistry Bipolar Electrode 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Schematic representation of the bipolar electrochemical cells designed Electrochemistry Bipolar Electrode 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research. Electrochemistry Bipolar Electrode.
From www.mdpi.com
Micromachines Free FullText Electrochemistry in a Two or Three Electrochemistry Bipolar Electrode A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has. Electrochemistry Bipolar Electrode.
From chemistry-europe.onlinelibrary.wiley.com
Closed Bipolar Electrode Array for Optical Reporting Reaction‐Coupled Electrochemistry Bipolar Electrode 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and. Electrochemistry Bipolar Electrode.
From www.researchgate.net
(a) A typical setup for bipolar electrochemistry experiment, with (b Electrochemistry Bipolar Electrode 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal. Electrochemistry Bipolar Electrode.
From www.mdpi.com
Metals Free FullText On the Application of Bipolar Electrochemistry Bipolar Electrode A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. In. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Scheme 1. Illustration of bipolar electrochemistry. Download Electrochemistry Bipolar Electrode Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a. Electrochemistry Bipolar Electrode.
From pubs.acs.org
Bipolar Electrode Arrays for Chemical Imaging and Multiplexed Sensing Electrochemistry Bipolar Electrode When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Schematic illustration of the principle of bipolar electrochemistry Electrochemistry Bipolar Electrode Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). When a sufficiently high electric field is applied. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Layout of the experimental rig based on a bipolar electrode Electrochemistry Bipolar Electrode Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. 1 the usual setup for bpe contains a pair of feeder electrodes. Electrochemistry Bipolar Electrode.
From www.researchgate.net
(a) Schematic illustration of the DCbipolar electrochemical system Electrochemistry Bipolar Electrode 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. Bipolar. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Electrochemical bipolar setup for the synthesis of ZnO (A), and an Electrochemistry Bipolar Electrode 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research. Electrochemistry Bipolar Electrode.
From www.researchgate.net
The segmented array setup for bipolar electrochemistry to measure local Electrochemistry Bipolar Electrode A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). When. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Schematic illustration of in situ Raman measurement along with bipolar Electrochemistry Bipolar Electrode A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. Bipolar electrochemistry (bpe) is an unconventional technique where. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Schematic illustration of the principle of bipolar electrochemistry Electrochemistry Bipolar Electrode Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes. Electrochemistry Bipolar Electrode.
From www.researchgate.net
(a) Schematic illustration of the electrochemical setup for ACbipolar Electrochemistry Bipolar Electrode When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. A bipolar electrode (bpe) is an electronic. Electrochemistry Bipolar Electrode.
From www.mdpi.com
Coatings Free FullText Electrochemical Characteristics with NaCl Electrochemistry Bipolar Electrode A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. 1 the usual setup for bpe contains a. Electrochemistry Bipolar Electrode.
From www.researchgate.net
(a) Schematic view of the bipolar electrochemical setup with the DVD Electrochemistry Bipolar Electrode Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. When a sufficiently high electric field is applied. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Schematic representation of the principle of bipolar electrochemistry Electrochemistry Bipolar Electrode In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is. Electrochemistry Bipolar Electrode.
From pubs.rsc.org
Wireless electrochemical actuation of soft materials towards chiral Electrochemistry Bipolar Electrode 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research. Electrochemistry Bipolar Electrode.
From www.researchgate.net
a) Schematic illustration of the bipolar electrochemistry. Reproduced Electrochemistry Bipolar Electrode Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Schematic representation of EC setup with monopolar electrodes in Electrochemistry Bipolar Electrode A different mode for driving electrochemical reactions has recently been proposed, in which bipolar electrodes (bpes). In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce electrochemical reactions at its extremities. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. When a sufficiently high electric field is. Electrochemistry Bipolar Electrode.
From www.mdpi.com
Biosensors Free FullText Newly Developed Electrochemiluminescence Electrochemistry Bipolar Electrode 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. In an electrochemical cell, a bipolar electrode (bpe) is a floating conductor that can induce. Electrochemistry Bipolar Electrode.
From www.researchgate.net
(a) Schematic setup of the bipolar electrochemistry experiment, and (b Electrochemistry Bipolar Electrode When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. Bipolar electrode corrosion (bpec), utilizing bipolar electrochemistry, has emerged as a pivotal technique in corrosion research by. 1 the usual setup for bpe contains. Electrochemistry Bipolar Electrode.
From www.researchgate.net
Schemes of electrochemical cells (a) with bipolar electrodes, (b) with Electrochemistry Bipolar Electrode A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. When a sufficiently high electric field is applied across the ionic phase, faradaic reactions occur at the ends of the bpe even though there is no direct electrical connection between it and an external power supply. Bipolar electrochemistry (bpe) is an unconventional technique where a. Electrochemistry Bipolar Electrode.
From www.researchgate.net
(PDF) Bipolar Electrochemistry A Powerful Tool for Electrifying Electrochemistry Bipolar Electrode A bipolar electrode (bpe) is an electronic conductor in contact with an ionically conductive phase. Bipolar electrochemistry (bpe) is an unconventional technique where a conducting object is addressed electrochemically in an. 1 the usual setup for bpe contains a pair of feeder electrodes physically connected with the both ends of a power supply and a conductive object (called as. Bipolar. Electrochemistry Bipolar Electrode.