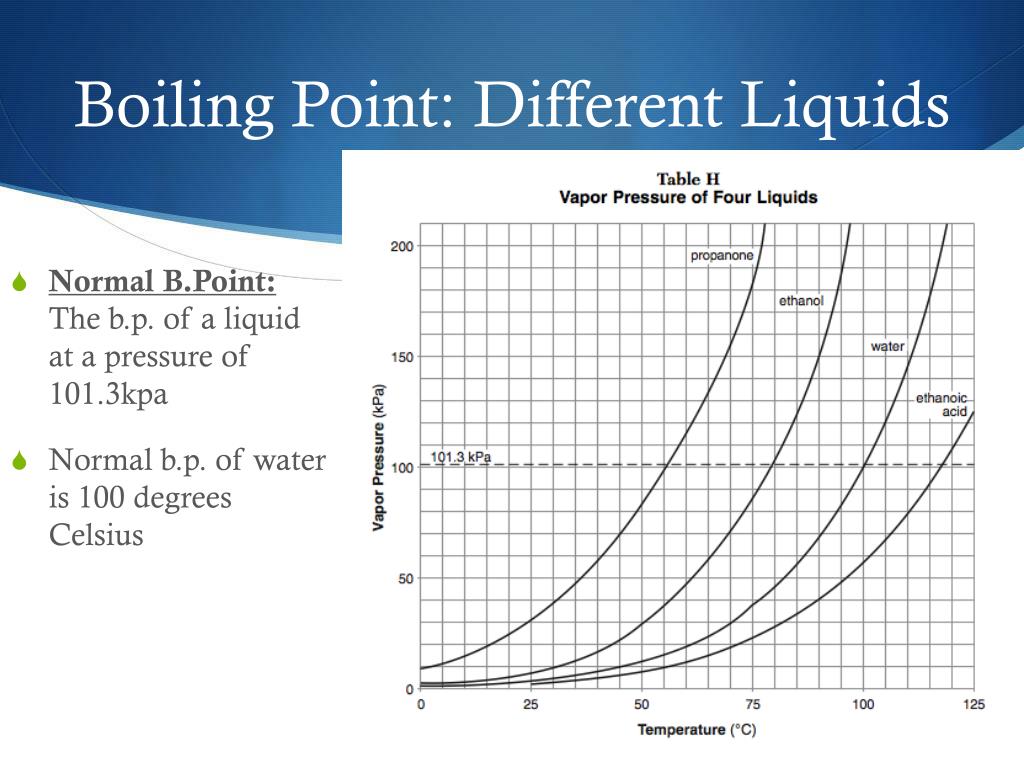
Why Does Increasing Pressure Increase Boiling Point . When a gas, the molecules occupy a far greater volume than they do when a liquid. In an open system this is called atmospheric. Therefore, increasing the pressure displaces the equilibrium (c.f. Factors that affect the boiling point. The intermolecular forces increase with increasing polarization of bonds. When the external pressure is: When the vapour pressure equals atmospheric pressure, the liquid boils. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Normally when we boil a liquid, we do so at atmospheric pressure. The pressure of gas above a liquid affects the boiling point. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). This is the boiling point which is usually quoted in chemical literature. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the.
from mavink.com
The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Factors that affect the boiling point. When a gas, the molecules occupy a far greater volume than they do when a liquid. This is the boiling point which is usually quoted in chemical literature. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). As elevation increases, atmospheric pressure decreases because air is less dense at higher. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. The pressure of gas above a liquid affects the boiling point. When the external pressure is:
Water Boiling Point Pressure Chart
Why Does Increasing Pressure Increase Boiling Point The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. When a gas, the molecules occupy a far greater volume than they do when a liquid. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. Normally when we boil a liquid, we do so at atmospheric pressure. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. Factors that affect the boiling point. Therefore, increasing the pressure displaces the equilibrium (c.f. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. In an open system this is called atmospheric. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The intermolecular forces increase with increasing polarization of bonds. The pressure of gas above a liquid affects the boiling point. Boiling point increases with molecular weight, and with surface area. When the vapour pressure equals atmospheric pressure, the liquid boils. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c).
From byjus.com
How does external pressure affect the boiling point Why Does Increasing Pressure Increase Boiling Point In an open system this is called atmospheric. Boiling point increases with molecular weight, and with surface area. When the vapour pressure equals atmospheric pressure, the liquid boils. Factors that affect the boiling point. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. When a. Why Does Increasing Pressure Increase Boiling Point.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Why Does Increasing Pressure Increase Boiling Point This is the boiling point which is usually quoted in chemical literature. When the vapour pressure equals atmospheric pressure, the liquid boils. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which. Why Does Increasing Pressure Increase Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Why Does Increasing Pressure Increase Boiling Point When a gas, the molecules occupy a far greater volume than they do when a liquid. Factors that affect the boiling point. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. The strength of intermolecular forces (and therefore impact on boiling points) is ionic >. Why Does Increasing Pressure Increase Boiling Point.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point Why Does Increasing Pressure Increase Boiling Point This is the boiling point which is usually quoted in chemical literature. When a gas, the molecules occupy a far greater volume than they do when a liquid. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Normally when we boil a liquid, we do so at atmospheric pressure. Factors that affect. Why Does Increasing Pressure Increase Boiling Point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Why Does Increasing Pressure Increase Boiling Point In an open system this is called atmospheric. When the external pressure is: The pressure of gas above a liquid affects the boiling point. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Boiling point increases with molecular weight, and with surface area. This is the boiling point which is usually quoted in chemical literature. Less. Why Does Increasing Pressure Increase Boiling Point.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Why Does Increasing Pressure Increase Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. When the vapour pressure equals atmospheric pressure, the liquid boils. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which. Why Does Increasing Pressure Increase Boiling Point.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Why Does Increasing Pressure Increase Boiling Point When a gas, the molecules occupy a far greater volume than they do when a liquid. The pressure of gas above a liquid affects the boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Therefore, increasing the pressure displaces. Why Does Increasing Pressure Increase Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart Why Does Increasing Pressure Increase Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. When the external pressure is: When a gas, the molecules occupy a far greater volume than they do when a liquid. Boiling point increases with molecular weight, and with surface area. The intermolecular forces increase with increasing polarization of bonds. Boiling point of a liquid can be increased. Why Does Increasing Pressure Increase Boiling Point.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Why Does Increasing Pressure Increase Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. When the external pressure is: Therefore, increasing the pressure displaces the equilibrium (c.f. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Factors that affect the boiling point. Boiling point of a liquid can be increased by increasing the external pressure on the liquid,. Why Does Increasing Pressure Increase Boiling Point.
From www.slideserve.com
PPT AP Chemistry Unit 2 PowerPoint Presentation, free download ID Why Does Increasing Pressure Increase Boiling Point When the vapour pressure equals atmospheric pressure, the liquid boils. This is the boiling point which is usually quoted in chemical literature. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. In an open system this is called atmospheric. Boiling point. Why Does Increasing Pressure Increase Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart Why Does Increasing Pressure Increase Boiling Point Therefore, increasing the pressure displaces the equilibrium (c.f. When a gas, the molecules occupy a far greater volume than they do when a liquid. The pressure of gas above a liquid affects the boiling point. Normally when we boil a liquid, we do so at atmospheric pressure. When the vapour pressure equals atmospheric pressure, the liquid boils. Less than one. Why Does Increasing Pressure Increase Boiling Point.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID Why Does Increasing Pressure Increase Boiling Point The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. In an open system this is called atmospheric. This is the boiling point which is usually quoted in chemical literature. When the vapour pressure equals atmospheric pressure, the liquid boils. Therefore, increasing the pressure displaces the equilibrium (c.f. Boiling. Why Does Increasing Pressure Increase Boiling Point.
From chem.libretexts.org
13.8 FreezingPoint Depression and BoilingPoint Elevation of Why Does Increasing Pressure Increase Boiling Point The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. The pressure of gas above a liquid affects the boiling point. This is the boiling point which is usually quoted in chemical literature. For example, an increase in pressure from 100 to 200 mmhg results in an increase in. Why Does Increasing Pressure Increase Boiling Point.
From preparatorychemistry.com
pH and Equilibrium Why Does Increasing Pressure Increase Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. The pressure of gas above a liquid affects the boiling point. Therefore, increasing the pressure displaces the equilibrium (c.f. Boiling point increases with molecular weight, and with surface area. When the external pressure is: Boiling point of a liquid can be increased by increasing the external pressure on. Why Does Increasing Pressure Increase Boiling Point.
From slidetodoc.com
Experiment 2 Boiling Point Determination The boiling point Why Does Increasing Pressure Increase Boiling Point When the external pressure is: For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). Normally when we boil a liquid, we do so at atmospheric pressure. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The intermolecular forces. Why Does Increasing Pressure Increase Boiling Point.
From mungfali.com
Water Boiling Point Pressure Chart Why Does Increasing Pressure Increase Boiling Point If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Normally when we boil a liquid, we do so at atmospheric pressure. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole >. Why Does Increasing Pressure Increase Boiling Point.
From www.youtube.com
Vapor Pressure and Boiling YouTube Why Does Increasing Pressure Increase Boiling Point When the vapour pressure equals atmospheric pressure, the liquid boils. The intermolecular forces increase with increasing polarization of bonds. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). Therefore, increasing the pressure displaces the equilibrium (c.f. As elevation increases, atmospheric pressure decreases. Why Does Increasing Pressure Increase Boiling Point.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Why Does Increasing Pressure Increase Boiling Point In an open system this is called atmospheric. When a gas, the molecules occupy a far greater volume than they do when a liquid. Factors that affect the boiling point. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. When the vapour pressure equals atmospheric. Why Does Increasing Pressure Increase Boiling Point.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co Why Does Increasing Pressure Increase Boiling Point Factors that affect the boiling point. Therefore, increasing the pressure displaces the equilibrium (c.f. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. Normally when we boil a liquid, we do so at atmospheric pressure. If this pressure is the standard pressure of 1 atm. Why Does Increasing Pressure Increase Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Why Does Increasing Pressure Increase Boiling Point For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Boiling point of a liquid can be increased by increasing the external pressure on the liquid,. Why Does Increasing Pressure Increase Boiling Point.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Does Increasing Pressure Increase Boiling Point If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. This is the boiling point which is usually quoted in chemical literature. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be. Why Does Increasing Pressure Increase Boiling Point.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5694866 Why Does Increasing Pressure Increase Boiling Point In an open system this is called atmospheric. This is the boiling point which is usually quoted in chemical literature. When the external pressure is: As elevation increases, atmospheric pressure decreases because air is less dense at higher. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred. Why Does Increasing Pressure Increase Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Why Does Increasing Pressure Increase Boiling Point Factors that affect the boiling point. When the vapour pressure equals atmospheric pressure, the liquid boils. The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. The pressure of gas above a liquid affects the boiling point. When the external pressure is: As elevation increases, atmospheric pressure decreases because. Why Does Increasing Pressure Increase Boiling Point.
From www.slideserve.com
PPT Energy, Temperature, Phase Changes PowerPoint Why Does Increasing Pressure Increase Boiling Point The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. The intermolecular. Why Does Increasing Pressure Increase Boiling Point.
From pakmcqs.com
With the increase of pressure, the boiling point of any substance Why Does Increasing Pressure Increase Boiling Point Factors that affect the boiling point. Boiling point increases with molecular weight, and with surface area. Normally when we boil a liquid, we do so at atmospheric pressure. When a gas, the molecules occupy a far greater volume than they do when a liquid. Therefore, increasing the pressure displaces the equilibrium (c.f. The strength of intermolecular forces (and therefore impact. Why Does Increasing Pressure Increase Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Why Does Increasing Pressure Increase Boiling Point Factors that affect the boiling point. This is the boiling point which is usually quoted in chemical literature. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. When a gas, the molecules occupy a far greater volume than they do when a liquid. The intermolecular forces increase with increasing polarization of bonds.. Why Does Increasing Pressure Increase Boiling Point.
From www.youtube.com
Boiling point and external pressure YouTube Why Does Increasing Pressure Increase Boiling Point The strength of intermolecular forces (and therefore impact on boiling points) is ionic > hydrogen bonding > dipole dipole > dispersion. The intermolecular forces increase with increasing polarization of bonds. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. Boiling point increases with molecular weight, and with surface area. When the external. Why Does Increasing Pressure Increase Boiling Point.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID Why Does Increasing Pressure Increase Boiling Point When the external pressure is: Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. This is the boiling point which is usually quoted in chemical literature. As elevation increases, atmospheric pressure decreases because air is less dense at higher. When a gas, the molecules occupy a far greater volume than they do. Why Does Increasing Pressure Increase Boiling Point.
From hxegzypap.blob.core.windows.net
Does Pressure Increase The Boiling Point at Edwin Dixon blog Why Does Increasing Pressure Increase Boiling Point The intermolecular forces increase with increasing polarization of bonds. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c).. Why Does Increasing Pressure Increase Boiling Point.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free Why Does Increasing Pressure Increase Boiling Point When the external pressure is: When a gas, the molecules occupy a far greater volume than they do when a liquid. In an open system this is called atmospheric. Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Boiling. Why Does Increasing Pressure Increase Boiling Point.
From www.worksheetsplanet.com
What is Boiling Point Why Does Increasing Pressure Increase Boiling Point When the vapour pressure equals atmospheric pressure, the liquid boils. Factors that affect the boiling point. Normally when we boil a liquid, we do so at atmospheric pressure. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from 52°c to 67°c). In an open system this. Why Does Increasing Pressure Increase Boiling Point.
From brainly.in
As pressure increase boiling point of liquid increase or decrease Why Does Increasing Pressure Increase Boiling Point Normally when we boil a liquid, we do so at atmospheric pressure. Factors that affect the boiling point. The intermolecular forces increase with increasing polarization of bonds. When the external pressure is: Less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point. The strength of intermolecular forces (and therefore impact on boiling points). Why Does Increasing Pressure Increase Boiling Point.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Why Does Increasing Pressure Increase Boiling Point In an open system this is called atmospheric. The pressure of gas above a liquid affects the boiling point. Boiling point increases with molecular weight, and with surface area. Factors that affect the boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its. Why Does Increasing Pressure Increase Boiling Point.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Why Does Increasing Pressure Increase Boiling Point When a gas, the molecules occupy a far greater volume than they do when a liquid. In an open system this is called atmospheric. Therefore, increasing the pressure displaces the equilibrium (c.f. Factors that affect the boiling point. Boiling point increases with molecular weight, and with surface area. This is the boiling point which is usually quoted in chemical literature.. Why Does Increasing Pressure Increase Boiling Point.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID Why Does Increasing Pressure Increase Boiling Point This is the boiling point which is usually quoted in chemical literature. In an open system this is called atmospheric. When a gas, the molecules occupy a far greater volume than they do when a liquid. For example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. Why Does Increasing Pressure Increase Boiling Point.