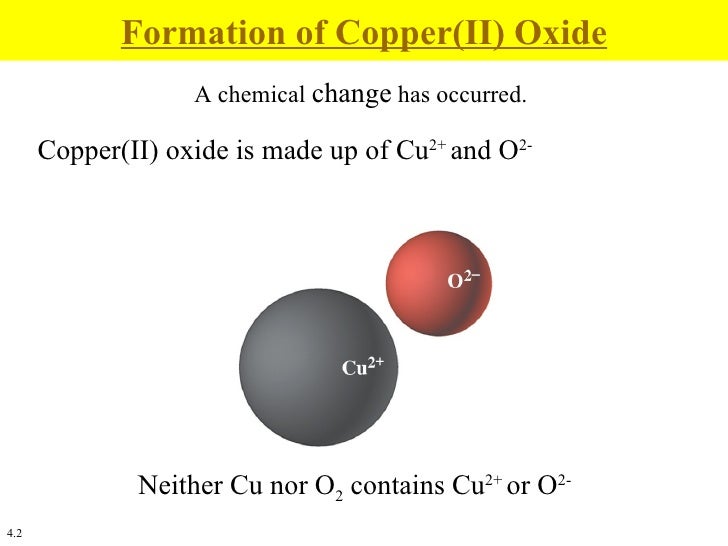
Copper Ii Oxide Chemical Equation . A black solid, it is one of the two stable oxides of copper, the. The formula for copper(ii) oxide is cuo. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. It has copper in its +2 oxidation state. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Its chemical formula is cuo.
from www.slideshare.net
In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. A black solid, it is one of the two stable oxides of copper, the. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. It has copper in its +2 oxidation state. Its chemical formula is cuo. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). The formula for copper(ii) oxide is cuo.
Chapter 4
Copper Ii Oxide Chemical Equation Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. The formula for copper(ii) oxide is cuo. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. It has copper in its +2 oxidation state. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Its chemical formula is cuo. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. A black solid, it is one of the two stable oxides of copper, the. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide.
From resource.studiaacademy.com
Chemical Formulae, Equations and Calculations Studia Academy Resources Copper Ii Oxide Chemical Equation The formula for copper(ii) oxide is cuo. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. Its chemical formula is cuo. It has copper in its +2. Copper Ii Oxide Chemical Equation.
From www.slideserve.com
PPT Copper Functioning as an Oxygen Carrier in Chemical Looping Copper Ii Oxide Chemical Equation Copper (ii) oxide, also known as cupric oxide, is a chemical compound. Its chemical formula is cuo. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). It has copper in its +2 oxidation. Copper Ii Oxide Chemical Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii Oxide Chemical Equation Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. The formula for copper(ii) oxide is cuo. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). It has copper in. Copper Ii Oxide Chemical Equation.
From www.chegg.com
Solved Correct PartC olid copper (II) oxide reacts with Copper Ii Oxide Chemical Equation Copper (ii) oxide, also known as cupric oxide, is a chemical compound. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). A black solid, it is one of the two stable. Copper Ii Oxide Chemical Equation.
From study.com
Copper II Oxide Formula, Properties & Structure Lesson Copper Ii Oxide Chemical Equation Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). The formula for copper(ii) oxide is cuo. A black solid, it is one of the two stable. Copper Ii Oxide Chemical Equation.
From cabinet.matttroy.net
Copper Oxide Symbol Periodic Table Matttroy Copper Ii Oxide Chemical Equation It has copper in its +2 oxidation state. The formula for copper(ii) oxide is cuo. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like. Copper Ii Oxide Chemical Equation.
From bnrc.springeropen.com
Copper(II) oxide nanocatalyst preparation and characterization green Copper Ii Oxide Chemical Equation As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Copper (ii) oxide, also known as cupric oxide, is a chemical. Copper Ii Oxide Chemical Equation.
From www.youtube.com
How to Balance CuO + H2 = Cu + H2O Copper (II) Oxide and Hydrogen Gas Copper Ii Oxide Chemical Equation It has copper in its +2 oxidation state. The formula for copper(ii) oxide is cuo. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. As the name suggests, copper (ii) oxide consists of one copper atom. Copper Ii Oxide Chemical Equation.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper Ii Oxide Chemical Equation Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. It has copper in its +2 oxidation state. Its chemical formula is cuo. The formula for copper(ii) oxide is cuo. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Ionic compounds like copper(ii) oxide are formed because the. Copper Ii Oxide Chemical Equation.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID4799899 Copper Ii Oxide Chemical Equation A black solid, it is one of the two stable oxides of copper, the. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Its chemical formula is cuo. The formula for copper(ii) oxide is cuo. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite. Copper Ii Oxide Chemical Equation.
From kristophernpetton.blogspot.com
Copper Oxide and Sulfuric Acid Formula KristophernPetton Copper Ii Oxide Chemical Equation In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. It has copper in its +2 oxidation state. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. If they weigh the reactants and products carefully, students can. Copper Ii Oxide Chemical Equation.
From www.slideserve.com
PPT Powerpoint to help with unit 22 PowerPoint Presentation, free Copper Ii Oxide Chemical Equation Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. A black solid, it is one of the two stable oxides of copper, the. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like. Copper Ii Oxide Chemical Equation.
From edu.rsc.org
Finding the formula of copper(II) oxide Experiment RSC Education Copper Ii Oxide Chemical Equation Copper (ii) oxide, also known as cupric oxide, is a chemical compound. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. It has copper in its +2 oxidation state. Its chemical formula is cuo. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper.. Copper Ii Oxide Chemical Equation.
From www.slideshare.net
Chapter 4 Copper Ii Oxide Chemical Equation As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). The formula for copper(ii) oxide is cuo. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. In this experiment, students heat. Copper Ii Oxide Chemical Equation.
From www.numerade.com
SOLVEDExample Write Out the balanced chemical equation for the Copper Ii Oxide Chemical Equation The formula for copper(ii) oxide is cuo. Its chemical formula is cuo. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom. Copper Ii Oxide Chemical Equation.
From testbook.com
Copper Oxide Learn Definition, Formula, Structure & Properties Copper Ii Oxide Chemical Equation In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Its chemical formula is cuo. A black solid,. Copper Ii Oxide Chemical Equation.
From www.toppr.com
Write balanced chemical equations for the following word equation Copper Ii Oxide Chemical Equation A black solid, it is one of the two stable oxides of copper, the. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). It has copper in its +2 oxidation state. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Ionic compounds like copper(ii) oxide are formed. Copper Ii Oxide Chemical Equation.
From www.toppr.com
On heating blue coloured powder of copper (II) nitrate in a boiling Copper Ii Oxide Chemical Equation A black solid, it is one of the two stable oxides of copper, the. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Copper (ii) oxide, also known as cupric oxide, is a chemical. Copper Ii Oxide Chemical Equation.
From www.chegg.com
Solved 1Give the chemical formula for copper (II) Copper Ii Oxide Chemical Equation It has copper in its +2 oxidation state. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and. Copper Ii Oxide Chemical Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper Ii Oxide Chemical Equation A black solid, it is one of the two stable oxides of copper, the. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). It has copper in its +2 oxidation state. The formula for copper(ii) oxide is cuo. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. Its. Copper Ii Oxide Chemical Equation.
From www.shutterstock.com
Copper Oxide Ii Chemical Formula Inside Stock Vector 549972436 Copper Ii Oxide Chemical Equation Its chemical formula is cuo. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. If they weigh the reactants and products carefully,. Copper Ii Oxide Chemical Equation.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Copper Ii Oxide Chemical Equation Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. The formula for copper(ii) oxide is cuo. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Its chemical formula is cuo. A black solid,. Copper Ii Oxide Chemical Equation.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Ii Oxide Chemical Equation As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). A black solid, it is one of the two stable oxides of copper, the. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Copper(ii) oxide or cupric oxide is. Copper Ii Oxide Chemical Equation.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Copper Ii Oxide Chemical Equation The formula for copper(ii) oxide is cuo. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. Its chemical formula is cuo. Copper (ii) oxide, also known as cupric oxide, is a. Copper Ii Oxide Chemical Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 = CuO + H2O YouTube Copper Ii Oxide Chemical Equation Its chemical formula is cuo. It has copper in its +2 oxidation state. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Copper(ii) oxide or cupric oxide is an inorganic compound with the. Copper Ii Oxide Chemical Equation.
From www.youtube.com
How to Calculate the Molar Mass of CuO Copper (II) oxide YouTube Copper Ii Oxide Chemical Equation Its chemical formula is cuo. A black solid, it is one of the two stable oxides of copper, the. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. Copper (ii) oxide, also known as cupric oxide,. Copper Ii Oxide Chemical Equation.
From studylib.net
90 Finding the formula of an oxide of copper Copper Ii Oxide Chemical Equation It has copper in its +2 oxidation state. A black solid, it is one of the two stable oxides of copper, the. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. The formula for copper(ii) oxide is cuo. Ionic. Copper Ii Oxide Chemical Equation.
From www.alamy.com
Fully labelled diagram showing the reduction of copper (II) oxide using Copper Ii Oxide Chemical Equation If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. The formula for copper(ii) oxide is cuo. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one. Copper Ii Oxide Chemical Equation.
From www.numerade.com
SOLVED Provide formulas, and a balanced equation for the following Copper Ii Oxide Chemical Equation Its chemical formula is cuo. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. It has copper in its +2 oxidation state. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. If they weigh the reactants and products carefully, students can then deduce the formula. Copper Ii Oxide Chemical Equation.
From www.numerade.com
Copper(II) sulfide is oxidized by molecular oxygen to produce gaseous Copper Ii Oxide Chemical Equation Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper.. Copper Ii Oxide Chemical Equation.
From www.numerade.com
SOLVED I am required to find the molecular equation, ionic equation Copper Ii Oxide Chemical Equation If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. It has copper in its +2 oxidation state. The formula for copper(ii) oxide is cuo. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. A black. Copper Ii Oxide Chemical Equation.
From www.answersarena.com
[Solved] What is the formula for copper(II) oxide? Express Copper Ii Oxide Chemical Equation A black solid, it is one of the two stable oxides of copper, the. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. Ionic compounds like copper(ii) oxide are. Copper Ii Oxide Chemical Equation.
From www.quanswer.com
1,what are the products of combining iron (2) and copper (2) oxide in a Copper Ii Oxide Chemical Equation It has copper in its +2 oxidation state. If they weigh the reactants and products carefully, students can then deduce the formula of the copper oxide. The formula for copper(ii) oxide is cuo. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together. Copper Ii Oxide Chemical Equation.
From www.slideshare.net
Chapter 4 Copper Ii Oxide Chemical Equation As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). A black solid, it is one of the two stable oxides of copper, the. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Its chemical formula is cuo. Ionic. Copper Ii Oxide Chemical Equation.
From www.fishersci.nl
Copper(II) oxide, 99.7 (metals basis), Thermo Scientific Chemicals Copper Ii Oxide Chemical Equation In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Copper (ii) oxide, also known as cupric oxide, is a chemical compound. Ionic compounds like copper(ii) oxide are formed because the oppositely charged ions stick together like opposite ends of two magnets. A black solid, it is one. Copper Ii Oxide Chemical Equation.