How Does The Ethylene Glycol Work . Its most common use is as an automotive antifreeze. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. A 1:1 solution of ethylene glycol and. This critical review describes a broad spectrum of properties of eg and significant advances in. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). The most important of these is 1,2. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. A glycol is an alcohol on. It is synthesized through the hydration of ethylene. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups.
from www.chegg.com
Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. A 1:1 solution of ethylene glycol and. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. This critical review describes a broad spectrum of properties of eg and significant advances in. Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. A glycol is an alcohol on. Its most common use is as an automotive antifreeze. The most important of these is 1,2.
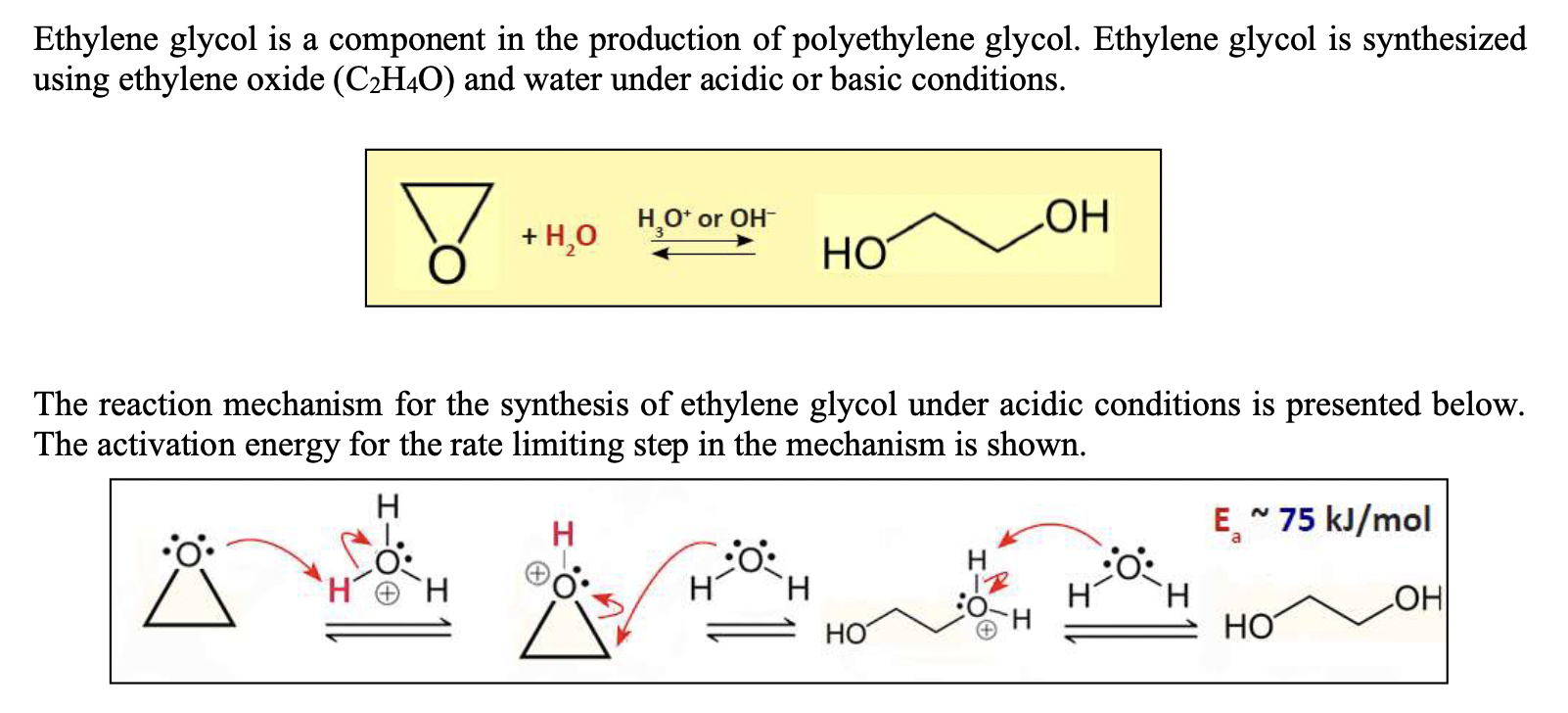
Solved Ethylene glycol is a component in the production of
How Does The Ethylene Glycol Work A glycol is an alcohol on. A glycol is an alcohol on. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). This critical review describes a broad spectrum of properties of eg and significant advances in. Its most common use is as an automotive antifreeze. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. A 1:1 solution of ethylene glycol and. The most important of these is 1,2. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. It is synthesized through the hydration of ethylene.
From www.maratekenvironmental.com
What is Glycol, and How Can it be Used as a Solvent? Maratek How Does The Ethylene Glycol Work Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). The most important of these is 1,2.. How Does The Ethylene Glycol Work.
From extractiongradesolvents.com
Ethylene Glycol Polyethylene Glycol Ethylene Glycol Uses How Does The Ethylene Glycol Work It is synthesized through the hydration of ethylene. The most important of these is 1,2. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c. How Does The Ethylene Glycol Work.
From www.researchgate.net
(PDF) Treatment of Ethylene Glycol Poisoning with Oral Ethyl Alcohol How Does The Ethylene Glycol Work The most important of these is 1,2. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. It is synthesized through the hydration of ethylene. Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. This. How Does The Ethylene Glycol Work.
From www.youtube.com
Glycol micro channel heat exchangers for data center free cooling How Does The Ethylene Glycol Work A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. A glycol is an alcohol on. Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. This critical review describes a broad spectrum of properties of. How Does The Ethylene Glycol Work.
From tschem.com.vn
Ethylene glycol là gì? Những điều liên quan đến Ethylene glycol How Does The Ethylene Glycol Work Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). This critical review describes a broad spectrum of properties of eg and significant advances in. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. It is synthesized through the hydration of ethylene. A glycol is an alcohol on. A. How Does The Ethylene Glycol Work.
From en.mehrnews.com
How to find Mono Ethylene Glycol manufacturer? Mehr News Agency How Does The Ethylene Glycol Work It is synthesized through the hydration of ethylene. Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). A glycol is an alcohol on. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient. How Does The Ethylene Glycol Work.
From rxmarine.com
ETHYLENE GLYCOL Manufacturer, Supplier, Exporter How Does The Ethylene Glycol Work Its most common use is as an automotive antifreeze. This critical review describes a broad spectrum of properties of eg and significant advances in. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. It is synthesized through the hydration of ethylene. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and. How Does The Ethylene Glycol Work.
From chemicalglossary.net
The Dangers of Ethylene Glycol Toxicity What You Need to Know How Does The Ethylene Glycol Work The most important of these is 1,2. A 1:1 solution of ethylene glycol and. A glycol is an alcohol on. This critical review describes a broad spectrum of properties of eg and significant advances in. Its most common use is as an automotive antifreeze. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an. How Does The Ethylene Glycol Work.
From www.glentham.com
Ethylene glycol (CAS 107211) Glentham Life Sciences How Does The Ethylene Glycol Work This critical review describes a broad spectrum of properties of eg and significant advances in. A 1:1 solution of ethylene glycol and. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. A glycol is an alcohol on. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene. How Does The Ethylene Glycol Work.
From www.globaldata.com
Ethylene Glycol Market Capacity & Capital Expenditure Analysis 2028 How Does The Ethylene Glycol Work Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). This critical review describes a broad spectrum of properties of eg and significant advances in. Ethylene glycol is a simple. How Does The Ethylene Glycol Work.
From journals.sagepub.com
Challenges in the diagnosis of ethylene glycol poisoning David J How Does The Ethylene Glycol Work Its most common use is as an automotive antifreeze. This critical review describes a broad spectrum of properties of eg and significant advances in. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. The most important of these is 1,2. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is. How Does The Ethylene Glycol Work.
From animalia-life.club
How Do You Treat Ethylene Glycol Poisoning In Dogs How Does The Ethylene Glycol Work It is synthesized through the hydration of ethylene. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. Its most common use is as an automotive antifreeze. This critical review describes a broad spectrum of properties of. How Does The Ethylene Glycol Work.
From www.degruyter.com
Synthesis of amphiphilic poly(ethylene glycol)blockpoly(methyl How Does The Ethylene Glycol Work Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). A 1:1 solution of ethylene glycol and. This critical review describes a broad spectrum of properties of eg and significant advances in. Ethylene glycol is a clear, viscous liquid primarily used. How Does The Ethylene Glycol Work.
From tschem.com.vn
Ethylene glycol là gì? Những điều liên quan đến Ethylene glycol How Does The Ethylene Glycol Work The most important of these is 1,2. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. A glycol is an alcohol on. Ethylene glycol is a simple diol, which is a. How Does The Ethylene Glycol Work.
From www.indochem.co.id
What is Ethylene Glycol or commonly called Monoethylene Glycol? Indochem How Does The Ethylene Glycol Work Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). The most important of these is 1,2. Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. A glycol is an alcohol on. A 1:1 solution of ethylene glycol and. Ethylene glycol is a simple diol, which is a type of. How Does The Ethylene Glycol Work.
From studylib.net
ethylene glycol How Does The Ethylene Glycol Work A glycol is an alcohol on. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. This critical review describes a broad spectrum of properties of eg and significant advances in. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). The most important of these is. How Does The Ethylene Glycol Work.
From 1malaysiabiolab.com
Ethylene Glycol 1Malaysia Bio Lab How Does The Ethylene Glycol Work A glycol is an alcohol on. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. It is synthesized through the hydration. How Does The Ethylene Glycol Work.
From www.slideserve.com
PPT Lectures 22 and 23 PowerPoint Presentation, free download ID How Does The Ethylene Glycol Work It is synthesized through the hydration of ethylene. A 1:1 solution of ethylene glycol and. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. This critical review describes a broad spectrum of properties of eg and significant advances in. A glycol is an alcohol on. Ethylene glycol is produced by reacting the. How Does The Ethylene Glycol Work.
From ar.inspiredpencil.com
Ethylene How Does The Ethylene Glycol Work Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. This critical review describes a broad spectrum of properties of eg and significant advances in. A 1:1 solution of ethylene glycol and. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. The most important of these is 1,2. Ethylene glycol is a. How Does The Ethylene Glycol Work.
From www.gz-supplies.com
The Ultimate Guide to Understanding the Difference Between Ethylene How Does The Ethylene Glycol Work A 1:1 solution of ethylene glycol and. A glycol is an alcohol on. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. It is synthesized through the hydration of ethylene. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. The. How Does The Ethylene Glycol Work.
From www.britannica.com
Solution polymerization chemistry Britannica How Does The Ethylene Glycol Work Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). The most important of these is 1,2. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. Ethylene glycol is produced by reacting. How Does The Ethylene Glycol Work.
From www.youtube.com
Medical vocabulary What does Ethylene Glycol mean YouTube How Does The Ethylene Glycol Work Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. A glycol is an alcohol on. The most important of these is 1,2. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Ethylene glycol is produced by reacting the chemical compound. How Does The Ethylene Glycol Work.
From www.animalpoisonline.co.uk
Ethylene glycol » Animal How Does The Ethylene Glycol Work A glycol is an alcohol on. Its most common use is as an automotive antifreeze. A 1:1 solution of ethylene glycol and. It is synthesized through the hydration of ethylene. Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. The most important of these is 1,2. Ethylene glycol is a clear, sweet, slightly viscous liquid that. How Does The Ethylene Glycol Work.
From stock.adobe.com
Ethylene glycol hand drawn vector formula chemical structure lettering How Does The Ethylene Glycol Work Its most common use is as an automotive antifreeze. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. It is synthesized through the hydration of ethylene. A 1:1 solution of ethylene. How Does The Ethylene Glycol Work.
From casereports.bmj.com
Lactate gap as a tool in identifying ethylene glycol poisoning BMJ How Does The Ethylene Glycol Work A glycol is an alcohol on. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). The most important of these is 1,2. It is synthesized through the hydration of ethylene. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. Its most common use is as. How Does The Ethylene Glycol Work.
From www.echemi.com
Buy Diethylene glycol monomethyl ether (DM) 99 Industrial Grade from How Does The Ethylene Glycol Work The most important of these is 1,2. A glycol is an alcohol on. It is synthesized through the hydration of ethylene. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Its most common use is as an automotive antifreeze. Ethylene glycol is produced by reacting. How Does The Ethylene Glycol Work.
From www.newsdayexpress.com
No matter how severe the cough is, do not drink this cough syrup, 18 How Does The Ethylene Glycol Work A glycol is an alcohol on. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). This critical review describes a broad spectrum of properties of eg and significant advances in. The most important of these is 1,2. It is. How Does The Ethylene Glycol Work.
From dokumen.tips
(PDF) Ethylene Glycol Poisoning med.nyu.edu · PDF fileEthylene Glycol How Does The Ethylene Glycol Work Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. Its most common use is as an automotive antifreeze. The most important of these is 1,2. Ethylene. How Does The Ethylene Glycol Work.
From en.wikipedia.org
Ethylene glycol poisoning Wikipedia How Does The Ethylene Glycol Work Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). A 1:1 solution of ethylene glycol and. The most important of these is 1,2. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Ethylene glycol is a simple diol,. How Does The Ethylene Glycol Work.
From www.youtube.com
Vapor pressure of ethylene glycol solution YouTube How Does The Ethylene Glycol Work Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). A 1:1 solution of ethylene glycol and. The most important of these is 1,2. It is synthesized through the hydration. How Does The Ethylene Glycol Work.
From www.animalia-life.club
Ethylene Glycol Structural Formula How Does The Ethylene Glycol Work Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications,. How Does The Ethylene Glycol Work.
From avatajhiz.com
اتیلن گلایکول (EXIR (Ethylene glycol آوا تجهیز آزما How Does The Ethylene Glycol Work Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. This critical review describes a broad spectrum of properties of eg and significant advances in. A glycol is an alcohol on. Its most common use is as. How Does The Ethylene Glycol Work.
From www.chegg.com
Solved Ethylene glycol is a component in the production of How Does The Ethylene Glycol Work Ethylene glycol is a simple diol, which is a type of alcohol that contains two hydroxyl groups. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). The most important of these is 1,2. This critical review describes a broad spectrum of properties of eg and significant advances in. Ethylene glycol is a clear,. How Does The Ethylene Glycol Work.
From chemicaldb.netlify.app
Propylene glycol poisoning How Does The Ethylene Glycol Work Ethylene glycol is produced by reacting the chemical compound ethylene oxide with water. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). A 1:1 solution of ethylene glycol and. A glycol is an alcohol on. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in. How Does The Ethylene Glycol Work.
From www.hpacmag.com
How to match glycol levels to various HVAC systems How Does The Ethylene Glycol Work Its most common use is as an automotive antifreeze. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, viscous liquid primarily used in industrial manufacturing applications, and is an ingredient in products like automotive antifreeze and coolant. Alcohols with two oh groups on adjacent carbon atoms are commonly known as glycols. Ethylene glycol is a simple diol,. How Does The Ethylene Glycol Work.