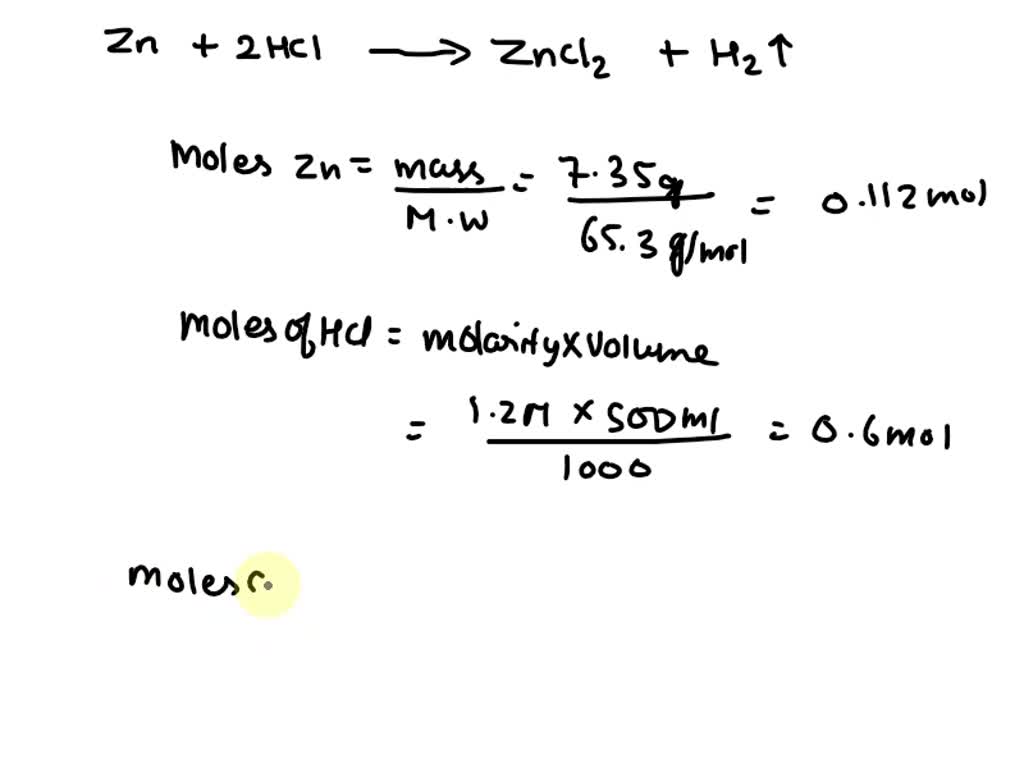What Dissolves Zinc . Galvanizing is the process of laying down a thin layer of zinc on. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: Most zinc today is obtained from zns,. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Water typically dissolves most ionic compounds and polar molecules. In aqueous solution the zn (ii) ion is present as. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Anionic znco 3 has a solubility of 0.21 g/l. The most important use of zinc today is in galvanizing other metals. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Examples of solubility of zinc compounds are:.
from www.numerade.com
Nonpolar molecules, such as those found in grease or oil, do not dissolve in. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Anionic znco 3 has a solubility of 0.21 g/l. In aqueous solution the zn (ii) ion is present as. Galvanizing is the process of laying down a thin layer of zinc on. Most zinc today is obtained from zns,. Examples of solubility of zinc compounds are:. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Water typically dissolves most ionic compounds and polar molecules.
SOLVED Zinc metal dissolves in hydrochloric acid to yield hydrogen gas
What Dissolves Zinc Galvanizing is the process of laying down a thin layer of zinc on. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Most zinc today is obtained from zns,. Galvanizing is the process of laying down a thin layer of zinc on. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: Anionic znco 3 has a solubility of 0.21 g/l. The most important use of zinc today is in galvanizing other metals. Water typically dissolves most ionic compounds and polar molecules. Examples of solubility of zinc compounds are:. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. In aqueous solution the zn (ii) ion is present as.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo What Dissolves Zinc The most important use of zinc today is in galvanizing other metals. In aqueous solution the zn (ii) ion is present as. Anionic znco 3 has a solubility of 0.21 g/l. Examples of solubility of zinc compounds are:. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Galvanizing is the process of laying down a. What Dissolves Zinc.
From www.numerade.com
SOLVEDZinc dissolves in hydrochloric acid to yield hydrogen gas (10 What Dissolves Zinc Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Most zinc today is obtained from zns,. In aqueous solution the zn (ii) ion is present as. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by. What Dissolves Zinc.
From www.doubtnut.com
Zinc dissolves in concentrated NaOH solution to produce dihydrogen as What Dissolves Zinc Water typically dissolves most ionic compounds and polar molecules. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Examples of solubility of zinc compounds are:. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. In aqueous solution. What Dissolves Zinc.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental What Dissolves Zinc The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Anionic znco 3 has a solubility of 0.21 g/l. Water typically dissolves most ionic compounds and polar molecules. Examples of solubility of. What Dissolves Zinc.
From www.chegg.com
Solved Zinc metal dissolves in aqueous hydrochloric acid to What Dissolves Zinc Anionic znco 3 has a solubility of 0.21 g/l. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Zinc dissolves in water as. What Dissolves Zinc.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube What Dissolves Zinc The most important use of zinc today is in galvanizing other metals. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Anionic znco 3 has a solubility of 0.21 g/l. Most zinc today is obtained from zns,. Zinc dissolves in water as znoh + (aq). What Dissolves Zinc.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) What Dissolves Zinc Water typically dissolves most ionic compounds and polar molecules. Galvanizing is the process of laying down a thin layer of zinc on. The most important use of zinc today is in galvanizing other metals. Anionic znco 3 has a solubility of 0.21 g/l. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Examples of solubility of zinc. What Dissolves Zinc.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells What Dissolves Zinc Water typically dissolves most ionic compounds and polar molecules. Examples of solubility of zinc compounds are:. Anionic znco 3 has a solubility of 0.21 g/l. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: The most important use of zinc today is in galvanizing other metals. Most zinc today is obtained from zns,. In aqueous solution the zn (ii) ion is. What Dissolves Zinc.
From www.youtube.com
What if you dissolve zinc in water? YouTube What Dissolves Zinc Anionic znco 3 has a solubility of 0.21 g/l. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). In aqueous solution the zn (ii) ion is present as. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by. What Dissolves Zinc.
From www.dreamstime.com
Illustration of Zinc Mineral Capsule Dissolves in the Stomach Stock What Dissolves Zinc Galvanizing is the process of laying down a thin layer of zinc on. Most zinc today is obtained from zns,. Water typically dissolves most ionic compounds and polar molecules. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: Examples of solubility of zinc compounds are:. Anionic znco 3 has a solubility of 0.21 g/l. Zinc metal dissolves slowly in dilute sulphuric. What Dissolves Zinc.
From www.youtube.com
Zinc metal reaction Nitric acid reaction Zinc in nitric acid What Dissolves Zinc Galvanizing is the process of laying down a thin layer of zinc on. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Water typically dissolves most ionic compounds and polar molecules. The most important use of zinc today is in galvanizing other metals. Examples of solubility of zinc compounds. What Dissolves Zinc.
From www.numerade.com
SOLVED Zinc metal dissolves in hydrochloric acid to yield hydrogen gas What Dissolves Zinc Water typically dissolves most ionic compounds and polar molecules. The most important use of zinc today is in galvanizing other metals. Galvanizing is the process of laying down a thin layer of zinc on. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). In aqueous solution the zn (ii) ion is present as. Zinc metal dissolves slowly. What Dissolves Zinc.
From edurev.in
The reaction of zinc with dilute and concentrated nitric acid What Dissolves Zinc Anionic znco 3 has a solubility of 0.21 g/l. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. In aqueous solution the zn (ii) ion is. What Dissolves Zinc.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper What Dissolves Zinc Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Water typically dissolves most ionic compounds and polar molecules. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: Anionic znco 3 has a solubility of 0.21 g/l. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. In aqueous solution the zn. What Dissolves Zinc.
From www.researchgate.net
Zinc vapor in BF bosh dissolves into molten iron. Download Scientific What Dissolves Zinc Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. The most important use of zinc today is in galvanizing other metals. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the. What Dissolves Zinc.
From www.walmart.com
EZ Melts Zinc 30 mg Vegan, Zero Sugar, 60 Fast Dissolve Tablets What Dissolves Zinc The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Most zinc today is obtained from zns,. Water typically dissolves most ionic compounds and polar molecules. Galvanizing is the. What Dissolves Zinc.
From www.chegg.com
Solved A piece of zinc metal dissolves completely in 50.00 What Dissolves Zinc Galvanizing is the process of laying down a thin layer of zinc on. The most important use of zinc today is in galvanizing other metals. Most zinc today is obtained from zns,. Examples of solubility of zinc compounds are:. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). The zinc(ii) hydroxide precipitate dissolves in excess ammonia: The. What Dissolves Zinc.
From woelen.homescience.net
Science made alive Chemistry/Elem/Elements/Zn What Dissolves Zinc In aqueous solution the zn (ii) ion is present as. Examples of solubility of zinc compounds are:. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Galvanizing is. What Dissolves Zinc.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 What Dissolves Zinc In aqueous solution the zn (ii) ion is present as. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Anionic znco 3 has a solubility of. What Dissolves Zinc.
From www.chegg.com
Solved A piece of zinc metal dissolves completely in 50.00 What Dissolves Zinc In aqueous solution the zn (ii) ion is present as. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Water typically dissolves most ionic compounds and polar molecules. Most zinc today is obtained from zns,. Galvanizing is. What Dissolves Zinc.
From questions.kunduz.com
3. From this description, identify the ph... Physical Chemistry What Dissolves Zinc Galvanizing is the process of laying down a thin layer of zinc on. Examples of solubility of zinc compounds are:. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. The most important use of zinc today is in galvanizing other metals. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: In aqueous solution. What Dissolves Zinc.
From www.numerade.com
SOLVED Zinc dissolves in hydrochloric acid to yield hydrogen gas as What Dissolves Zinc Galvanizing is the process of laying down a thin layer of zinc on. Examples of solubility of zinc compounds are:. The most important use of zinc today is in galvanizing other metals. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or. What Dissolves Zinc.
From www.youtube.com
The reaction of Zinc(Zn) and Dilute Sulphuric Acid(H2SO4) YouTube What Dissolves Zinc Galvanizing is the process of laying down a thin layer of zinc on. Most zinc today is obtained from zns,. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). The zinc(ii) hydroxide precipitate dissolves in excess ammonia: Nonpolar molecules, such. What Dissolves Zinc.
From www.numerade.com
SOLVED Zinc dissolves in hydrochloric acid to yield hydrogen gas as What Dissolves Zinc Galvanizing is the process of laying down a thin layer of zinc on. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Most zinc today is obtained from zns,. In aqueous solution the zn (ii) ion is present as. The most important use of zinc today is in galvanizing other metals. Zinc dissolves in water. What Dissolves Zinc.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate Chemistry What Dissolves Zinc Anionic znco 3 has a solubility of 0.21 g/l. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Examples of solubility of zinc compounds are:. Most zinc today is obtained from zns,. The most important use of zinc today is in galvanizing other metals. Water typically dissolves most ionic compounds and polar molecules. Zinc dissolves. What Dissolves Zinc.
From www.vitabiotics.com
The Best Sources Of Zinc What Dissolves Zinc Water typically dissolves most ionic compounds and polar molecules. Examples of solubility of zinc compounds are:. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. In aqueous solution the zn (ii) ion is present as. Zinc metal dissolves slowly in dilute sulphuric acid to form. What Dissolves Zinc.
From www.chegg.com
Solved A student wants to dissolve zinc metal ( Zn in a What Dissolves Zinc Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. In aqueous solution the zn (ii) ion is present as. The most important use of zinc today is in galvanizing other metals. Galvanizing is the process of laying down. What Dissolves Zinc.
From www.walmart.com
Spring Valley Fast Dissolve Zinc with Vitamin C, Chewable Tablets What Dissolves Zinc Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Most zinc today is obtained from zns,. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc. What Dissolves Zinc.
From www.chegg.com
Solved QUESTION 7 Zinc dissolves in hydrochloric acid to What Dissolves Zinc Water typically dissolves most ionic compounds and polar molecules. Anionic znco 3 has a solubility of 0.21 g/l. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. The bath is supplied with zinc cations either by zinc anodes,. What Dissolves Zinc.
From zonebutterworthtb.z21.web.core.windows.net
Chemical Reactions With Zinc What Dissolves Zinc The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Examples of solubility of zinc compounds are:. In aqueous solution the zn (ii) ion is present as. Most zinc today is obtained from zns,. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii). What Dissolves Zinc.
From www.chegg.com
Solved Zinc dissolves in hydrochloric acid to yield hydrogen What Dissolves Zinc Galvanizing is the process of laying down a thin layer of zinc on. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: Examples of solubility of zinc compounds are:. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved. What Dissolves Zinc.
From www.chegg.com
Solved 6) Zinc dissolves in hydrochloric acid to yield What Dissolves Zinc Anionic znco 3 has a solubility of 0.21 g/l. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Water typically dissolves most ionic compounds and polar molecules. In aqueous solution the zn (ii) ion is present as. Galvanizing is the process of laying down a thin layer of zinc on. The bath is supplied with zinc cations. What Dissolves Zinc.
From www.researchgate.net
Zinc vapor in BF bosh dissolves into molten iron. Download Scientific What Dissolves Zinc The zinc(ii) hydroxide precipitate dissolves in excess ammonia: Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Water typically dissolves most ionic compounds and polar molecules. Most zinc today is obtained from zns,. Anionic znco 3 has a solubility of 0.21 g/l. Examples of solubility of zinc compounds are:. Zinc metal dissolves slowly in dilute. What Dissolves Zinc.
From www.chegg.com
15.71 Zinc metal dissolves in hydrochloric acid What Dissolves Zinc Water typically dissolves most ionic compounds and polar molecules. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. The zinc(ii) hydroxide precipitate dissolves in excess ammonia: Galvanizing is the process of laying down a thin layer of zinc on. In aqueous solution the zn (ii) ion is present as. Zinc metal dissolves slowly in dilute. What Dissolves Zinc.
From www.haikudeck.com
Zinc by Corrine B What Dissolves Zinc The most important use of zinc today is in galvanizing other metals. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously. Galvanizing is the process of laying down a thin layer of zinc on. Zinc dissolves in water as znoh + (aq) or zn 2+. What Dissolves Zinc.