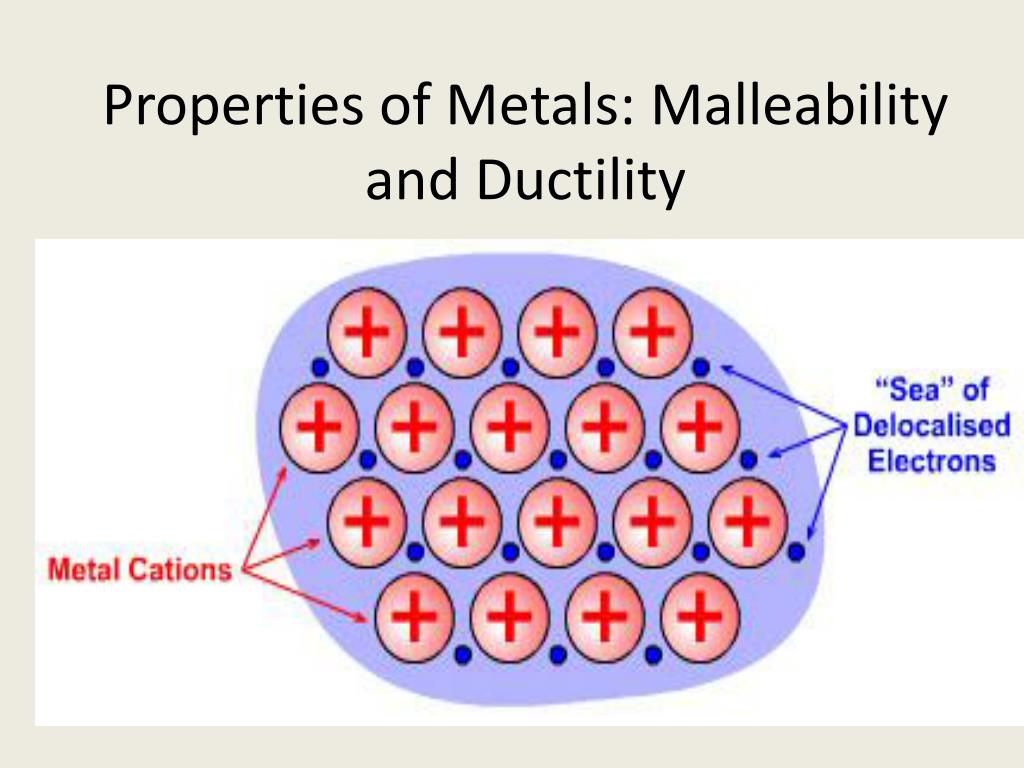
Metals Are Malleable Bc The Metallic Bonding . Metallic bonds are very strong so the giant metallic structure is strongly held together: The bonding that occurs in a metal is responsible for its distinctive properties: Metallic bonds are very strong so the giant metallic structure is strongly held together: Gold, silver, aluminum, iron, and copper are malleable. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Luster, malleability, ductility, and excellent conductivity. Metals are malleable and ductile. Layers of positive ions can slide over one another and take up different positions. A metal that you can hammer into thin sheets is malleable. This is because they consist of layers of ions that can slide over one.
from www.slideserve.com
A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. The bonding that occurs in a metal is responsible for its distinctive properties: A metal that you can hammer into thin sheets is malleable. This is because they consist of layers of ions that can slide over one. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Metals are malleable and ductile. Gold, silver, aluminum, iron, and copper are malleable. Metallic bonds are very strong so the giant metallic structure is strongly held together: Luster, malleability, ductility, and excellent conductivity. Layers of positive ions can slide over one another and take up different positions.
PPT Objectives PowerPoint Presentation, free download ID5858440
Metals Are Malleable Bc The Metallic Bonding Metallic bonds are very strong so the giant metallic structure is strongly held together: This is because they consist of layers of ions that can slide over one. Metallic bonds are very strong so the giant metallic structure is strongly held together: Layers of positive ions can slide over one another and take up different positions. Metals are malleable and ductile. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The bonding that occurs in a metal is responsible for its distinctive properties: Metallic bonds are very strong so the giant metallic structure is strongly held together: A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Gold, silver, aluminum, iron, and copper are malleable. A metal that you can hammer into thin sheets is malleable. Luster, malleability, ductility, and excellent conductivity.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID5858440 Metals Are Malleable Bc The Metallic Bonding Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Luster, malleability, ductility, and excellent conductivity. Metallic bonds are very strong so the giant metallic structure is strongly held together: Layers of positive ions can slide over one another and take up different positions. A sheet of aluminum foil and a copper wire are. Metals Are Malleable Bc The Metallic Bonding.
From www.science-revision.co.uk
Metallic bonding Metals Are Malleable Bc The Metallic Bonding The bonding that occurs in a metal is responsible for its distinctive properties: Metallic bonds are very strong so the giant metallic structure is strongly held together: Luster, malleability, ductility, and excellent conductivity. This is because they consist of layers of ions that can slide over one. A metal that you can hammer into thin sheets is malleable. Gold, silver,. Metals Are Malleable Bc The Metallic Bonding.
From www.youtube.com
Metallic Bonding conductivity, malleability and high mp YouTube Metals Are Malleable Bc The Metallic Bonding Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Luster, malleability, ductility, and excellent conductivity. Layers of positive ions can slide over one another and take up different positions. Gold, silver, aluminum, iron, and copper are malleable. A sheet of aluminum foil and a copper wire are both places where you can see. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4982303 Metals Are Malleable Bc The Metallic Bonding Luster, malleability, ductility, and excellent conductivity. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Metallic bonds are very strong so the giant metallic structure is strongly held together: Layers of positive ions can slide over one another and take up different positions. The bonding that occurs in a. Metals Are Malleable Bc The Metallic Bonding.
From ar.inspiredpencil.com
Metallic Bonds Malleable Metals Are Malleable Bc The Metallic Bonding Gold, silver, aluminum, iron, and copper are malleable. Luster, malleability, ductility, and excellent conductivity. The bonding that occurs in a metal is responsible for its distinctive properties: Metals are malleable and ductile. Layers of positive ions can slide over one another and take up different positions. A sheet of aluminum foil and a copper wire are both places where you. Metals Are Malleable Bc The Metallic Bonding.
From slideplayer.com
Metallic Bonds. ppt download Metals Are Malleable Bc The Metallic Bonding Gold, silver, aluminum, iron, and copper are malleable. A metal that you can hammer into thin sheets is malleable. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metallic bonds are very strong so the giant metallic structure is strongly held together: The bonding that occurs in a metal is responsible for its distinctive properties:. Metals Are Malleable Bc The Metallic Bonding.
From slideplayer.com
KS4 Chemistry Metallic Bonding. ppt download Metals Are Malleable Bc The Metallic Bonding Luster, malleability, ductility, and excellent conductivity. Metallic bonds are very strong so the giant metallic structure is strongly held together: A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Gold, silver, aluminum, iron, and copper are malleable. The bonding that occurs in a metal is responsible for its distinctive. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Metallic Bond PowerPoint Presentation, free download ID438230 Metals Are Malleable Bc The Metallic Bonding Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Gold, silver, aluminum, iron, and copper are malleable. This is because they consist of layers of ions that can slide over one. The bonding that occurs in a metal is responsible for its distinctive properties: Metallic bonds are very strong so the giant metallic. Metals Are Malleable Bc The Metallic Bonding.
From acechemistry.co.uk
Metallic bonding and metallic structures Ace Chemistry Metals Are Malleable Bc The Metallic Bonding Metals are malleable and ductile. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metallic bonds are very strong so the giant metallic structure is strongly held together: A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Metals tend to have high melting points. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Metals PowerPoint Presentation ID1462875 Metals Are Malleable Bc The Metallic Bonding A metal that you can hammer into thin sheets is malleable. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Metallic bonds are very strong so the giant metallic structure is strongly held together: The bonding that occurs in. Metals Are Malleable Bc The Metallic Bonding.
From www.science-revision.co.uk
Metallic bonding Metals Are Malleable Bc The Metallic Bonding A metal that you can hammer into thin sheets is malleable. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Gold, silver, aluminum, iron, and copper are malleable. Metals are malleable and ductile. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action.. Metals Are Malleable Bc The Metallic Bonding.
From slidetodoc.com
Metallic Bonding and Structure Describe metallic bonding as Metals Are Malleable Bc The Metallic Bonding This is because they consist of layers of ions that can slide over one. Layers of positive ions can slide over one another and take up different positions. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metals are malleable and ductile. Gold, silver, aluminum, iron, and copper are malleable. A sheet of aluminum foil. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT 4.4 Metallic bonding PowerPoint Presentation ID400526 Metals Are Malleable Bc The Metallic Bonding This is because they consist of layers of ions that can slide over one. Gold, silver, aluminum, iron, and copper are malleable. Metals are malleable and ductile. Luster, malleability, ductility, and excellent conductivity. Metallic bonds are very strong so the giant metallic structure is strongly held together: The bonding that occurs in a metal is responsible for its distinctive properties:. Metals Are Malleable Bc The Metallic Bonding.
From slideplayer.com
Chemical Bonding Chapter ppt download Metals Are Malleable Bc The Metallic Bonding Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The bonding that occurs in a metal is responsible for its distinctive properties: Metals are malleable and ductile. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Metallic bonds are very strong so. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID2696346 Metals Are Malleable Bc The Metallic Bonding Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metals are malleable and ductile. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. This is because they consist. Metals Are Malleable Bc The Metallic Bonding.
From online-learning-college.com
Metallic bonding What is it? Properties of metallic structures Metals Are Malleable Bc The Metallic Bonding Luster, malleability, ductility, and excellent conductivity. Metallic bonds are very strong so the giant metallic structure is strongly held together: Layers of positive ions can slide over one another and take up different positions. This is because they consist of layers of ions that can slide over one. Gold, silver, aluminum, iron, and copper are malleable. Metallic bonds are very. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Metallic bonding and properties PowerPoint Presentation ID3072363 Metals Are Malleable Bc The Metallic Bonding Metals are malleable and ductile. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Gold, silver, aluminum, iron, and copper are malleable. Metallic bonds are very strong so the giant metallic structure is strongly held together: A metal that you can hammer into thin sheets is malleable. This is because they consist of. Metals Are Malleable Bc The Metallic Bonding.
From chemnotcheem.com
Metallic bonding & giant metallic structure O Level Chemistry Notes Metals Are Malleable Bc The Metallic Bonding Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Metallic bonds are very strong so the giant metallic structure is strongly held together: A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Metals are malleable and ductile. A metal that you can. Metals Are Malleable Bc The Metallic Bonding.
From slideplayer.com
Chapter 7 & 8 Ions and Bonding. ppt download Metals Are Malleable Bc The Metallic Bonding Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The bonding that occurs in a metal is responsible for its distinctive properties: Metals are malleable and ductile. Layers of positive ions can slide over one another and take up different positions. Metallic bonds are very strong so the giant metallic structure is strongly. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Bonding in Metals PowerPoint Presentation, free download ID4819941 Metals Are Malleable Bc The Metallic Bonding Gold, silver, aluminum, iron, and copper are malleable. Metallic bonds are very strong so the giant metallic structure is strongly held together: This is because they consist of layers of ions that can slide over one. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. The bonding that occurs. Metals Are Malleable Bc The Metallic Bonding.
From slideplayer.com
Metallic Bonding Topic ppt download Metals Are Malleable Bc The Metallic Bonding This is because they consist of layers of ions that can slide over one. Metals are malleable and ductile. Gold, silver, aluminum, iron, and copper are malleable. Metallic bonds are very strong so the giant metallic structure is strongly held together: A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in. Metals Are Malleable Bc The Metallic Bonding.
From slideplayer.com
Ionic & Metallic Bonding ppt download Metals Are Malleable Bc The Metallic Bonding Luster, malleability, ductility, and excellent conductivity. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The bonding that occurs in a metal is responsible for its distinctive properties: Metals are malleable and ductile. Layers of positive ions can slide over one another and take up different positions. A sheet of aluminum foil and. Metals Are Malleable Bc The Metallic Bonding.
From slideplayer.com
Ch. 6.4 Bonding in Metals Metallic Bonding. ppt download Metals Are Malleable Bc The Metallic Bonding This is because they consist of layers of ions that can slide over one. The bonding that occurs in a metal is responsible for its distinctive properties: Metals are malleable and ductile. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Metallic bonds are very strong so the giant. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID9555729 Metals Are Malleable Bc The Metallic Bonding The bonding that occurs in a metal is responsible for its distinctive properties: Gold, silver, aluminum, iron, and copper are malleable. Metallic bonds are very strong so the giant metallic structure is strongly held together: Luster, malleability, ductility, and excellent conductivity. Layers of positive ions can slide over one another and take up different positions. Metals tend to have high. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Metallic bonding and properties PowerPoint Presentation, free Metals Are Malleable Bc The Metallic Bonding Metallic bonds are very strong so the giant metallic structure is strongly held together: Metallic bonds are very strong so the giant metallic structure is strongly held together: Metals are malleable and ductile. The bonding that occurs in a metal is responsible for its distinctive properties: Luster, malleability, ductility, and excellent conductivity. Metals tend to have high melting points and. Metals Are Malleable Bc The Metallic Bonding.
From quizlet.com
Chemistry C2 Metallic Bonding Diagram Quizlet Metals Are Malleable Bc The Metallic Bonding A metal that you can hammer into thin sheets is malleable. This is because they consist of layers of ions that can slide over one. The bonding that occurs in a metal is responsible for its distinctive properties: Layers of positive ions can slide over one another and take up different positions. Metals tend to have high melting points and. Metals Are Malleable Bc The Metallic Bonding.
From slideplayer.com
Metallic Bonding. ppt download Metals Are Malleable Bc The Metallic Bonding The bonding that occurs in a metal is responsible for its distinctive properties: A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Luster, malleability, ductility, and excellent conductivity. This is because they consist. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Metallic Bonding and Naming of Ionic Bonds PowerPoint Metals Are Malleable Bc The Metallic Bonding Luster, malleability, ductility, and excellent conductivity. A metal that you can hammer into thin sheets is malleable. Metallic bonds are very strong so the giant metallic structure is strongly held together: The bonding that occurs in a metal is responsible for its distinctive properties: Metallic bonds are very strong so the giant metallic structure is strongly held together: Gold, silver,. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID2041377 Metals Are Malleable Bc The Metallic Bonding This is because they consist of layers of ions that can slide over one. Gold, silver, aluminum, iron, and copper are malleable. Luster, malleability, ductility, and excellent conductivity. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The bonding. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID2696346 Metals Are Malleable Bc The Metallic Bonding A metal that you can hammer into thin sheets is malleable. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. This is because they consist of layers of ions that can slide over one. Metallic bonds are very strong so the giant metallic structure is strongly held together: The. Metals Are Malleable Bc The Metallic Bonding.
From www.slideshare.net
Metallic Bonding Metals Are Malleable Bc The Metallic Bonding Luster, malleability, ductility, and excellent conductivity. The bonding that occurs in a metal is responsible for its distinctive properties: Gold, silver, aluminum, iron, and copper are malleable. A metal that you can hammer into thin sheets is malleable. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metals tend to have high melting points and. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Chapter 12 Elements and Their Properties PowerPoint Presentation Metals Are Malleable Bc The Metallic Bonding Luster, malleability, ductility, and excellent conductivity. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metallic bonds are very strong so the giant metallic structure is strongly held together: The bonding that occurs in a metal is responsible for its distinctive properties: Gold, silver, aluminum, iron, and copper are malleable. A sheet of aluminum foil. Metals Are Malleable Bc The Metallic Bonding.
From www.slideserve.com
PPT Metallic bonding and properties PowerPoint Presentation, free Metals Are Malleable Bc The Metallic Bonding This is because they consist of layers of ions that can slide over one. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metallic bonds are very strong so the giant metallic structure is strongly held together: A metal that you can hammer into thin sheets is malleable. Luster, malleability, ductility, and excellent conductivity. Metals. Metals Are Malleable Bc The Metallic Bonding.
From www.youtube.com
Metallic Bond Properties & EN of Ionic & Covalent Bonds YouTube Metals Are Malleable Bc The Metallic Bonding A metal that you can hammer into thin sheets is malleable. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Luster, malleability, ductility, and excellent conductivity. Layers of positive ions can slide over one another and take up different positions. Metals are malleable and ductile. Gold, silver, aluminum, iron,. Metals Are Malleable Bc The Metallic Bonding.
From www.thesciencehive.co.uk
Metallic Bonding (GCSE) — the science sauce Metals Are Malleable Bc The Metallic Bonding Metallic bonds are very strong so the giant metallic structure is strongly held together: This is because they consist of layers of ions that can slide over one. Gold, silver, aluminum, iron, and copper are malleable. Metallic bonds are very strong so the giant metallic structure is strongly held together: A metal that you can hammer into thin sheets is. Metals Are Malleable Bc The Metallic Bonding.