What Happens When You Mix Oxygen And Hydrogen . But if you add some energy, say from a match, the gases will break. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. This is a synthesis reaction that makes water from its elements. Hydrogen and oxygen arent both gasses, they are both elements. If you combine oxygen gas and hydrogen gas, you'll still have gas. It’s also a combustion reaction. The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. Liquid hydrogen and liquid oxygen both exist. How do two gases combine to make liquid water? 2 h 2 + o 2 → 2 h 2 o. The balanced equation for the chemical reaction is: Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts.
from www.alamy.com
The balanced equation for the chemical reaction is: 2 h 2 + o 2 → 2 h 2 o. How do two gases combine to make liquid water? Liquid hydrogen and liquid oxygen both exist. The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. But if you add some energy, say from a match, the gases will break. This is a synthesis reaction that makes water from its elements. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. It’s also a combustion reaction. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts.
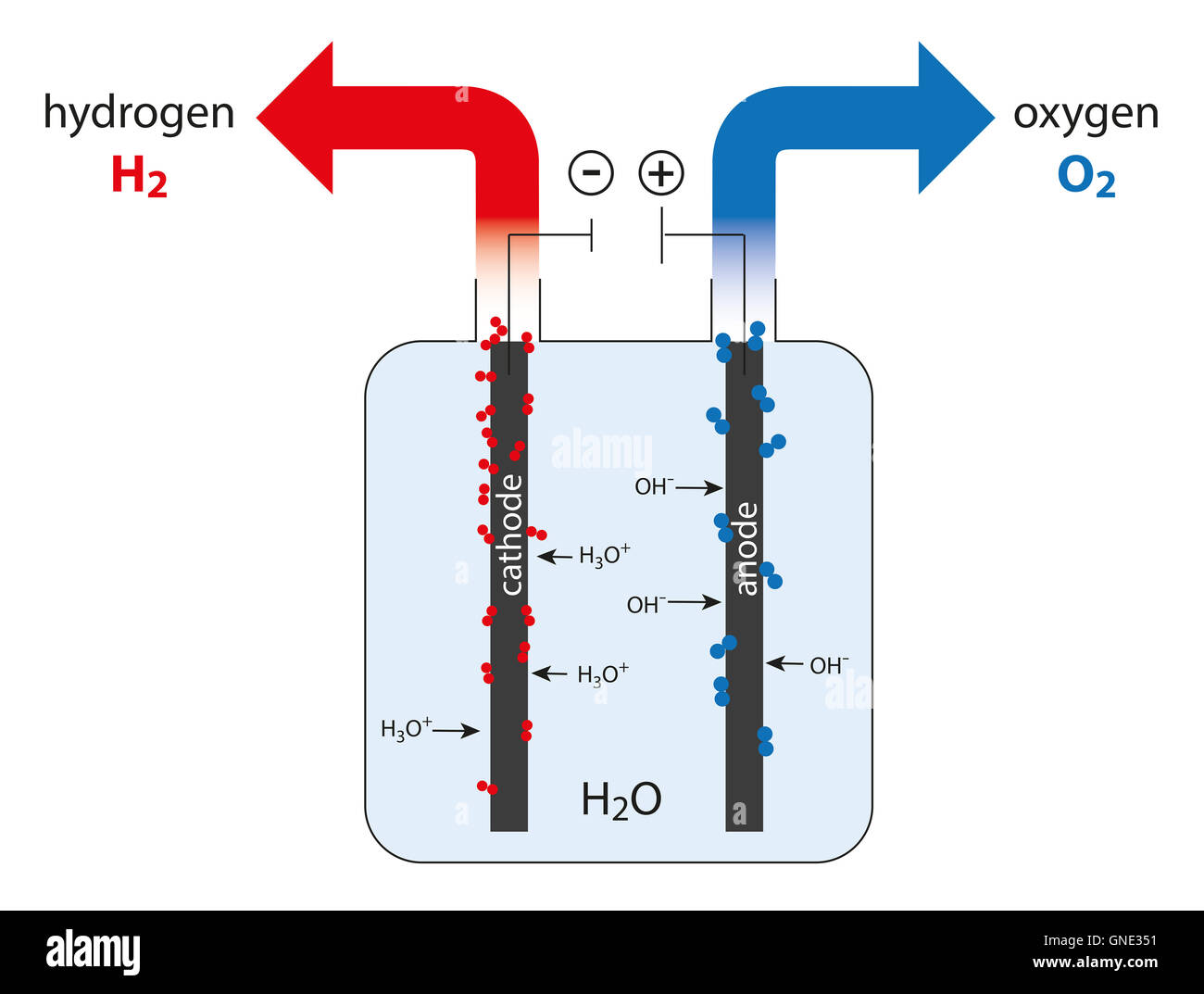
electrolysis cell of water to produce hydrogen and oxygen Stock Photo
What Happens When You Mix Oxygen And Hydrogen The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. How do two gases combine to make liquid water? Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. 2 h 2 + o 2 → 2 h 2 o. Liquid hydrogen and liquid oxygen both exist. But if you add some energy, say from a match, the gases will break. This is a synthesis reaction that makes water from its elements. The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. The balanced equation for the chemical reaction is: If you combine oxygen gas and hydrogen gas, you'll still have gas. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. Hydrogen and oxygen arent both gasses, they are both elements. It’s also a combustion reaction.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer What Happens When You Mix Oxygen And Hydrogen This is a synthesis reaction that makes water from its elements. It’s also a combustion reaction. Hydrogen and oxygen arent both gasses, they are both elements. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. But if you add some energy, say from a match, the gases will break. Making water from hydrogen and oxygen is. What Happens When You Mix Oxygen And Hydrogen.
From www.youtube.com
3.1 Hydrogen, Oxygen, & Water YouTube What Happens When You Mix Oxygen And Hydrogen Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. This is a synthesis reaction that makes water from its elements. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. It’s also a combustion reaction. How do two gases combine to make liquid water?. What Happens When You Mix Oxygen And Hydrogen.
From www.hhocarfuelcell.com
How to split water into hydrogen and oxygen at home HHO What Happens When You Mix Oxygen And Hydrogen But if you add some energy, say from a match, the gases will break. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. This is a synthesis reaction that makes water from its elements. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat.. What Happens When You Mix Oxygen And Hydrogen.
From www.slideserve.com
PPT Fuel cells and the Reaction of Oxygen and Hydrogen PowerPoint What Happens When You Mix Oxygen And Hydrogen Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. This is a synthesis reaction that makes water from its elements. How do two gases combine to make liquid water? The balanced equation for the. What Happens When You Mix Oxygen And Hydrogen.
From www.slideserve.com
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183 What Happens When You Mix Oxygen And Hydrogen It’s also a combustion reaction. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. But if you add some energy, say from a match, the gases will break. Liquid hydrogen and liquid oxygen both exist. Hydrogen and oxygen arent both gasses, they are both elements. 2 h 2 + o 2 → 2 h 2 o.. What Happens When You Mix Oxygen And Hydrogen.
From byjus.com
a jar has mixture of hydrogen and oxygen gases in the ratio 1is to 5 What Happens When You Mix Oxygen And Hydrogen But if you add some energy, say from a match, the gases will break. It’s also a combustion reaction. If you combine oxygen gas and hydrogen gas, you'll still have gas. The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. Hydrogen and oxygen arent both gasses,. What Happens When You Mix Oxygen And Hydrogen.
From www.vecteezy.com
Electrolysis of water forming Hydrogen and Oxygen vector illustration What Happens When You Mix Oxygen And Hydrogen Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. 2 h 2 + o 2 → 2 h 2 o. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. Hydrogen and oxygen arent both gasses, they are both elements. It’s also a combustion. What Happens When You Mix Oxygen And Hydrogen.
From www.differencebetween.com
Difference Between Hydrogen and Oxygen Hydrogen vs Oxygen What Happens When You Mix Oxygen And Hydrogen This is a synthesis reaction that makes water from its elements. But if you add some energy, say from a match, the gases will break. The balanced equation for the chemical reaction is: The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. If you combine oxygen. What Happens When You Mix Oxygen And Hydrogen.
From www.sciencephoto.com
Reaction of hydrogen and oxygen to water Stock Image C017/3607 What Happens When You Mix Oxygen And Hydrogen This is a synthesis reaction that makes water from its elements. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. But if you add some energy, say from a match, the gases will break. Liquid hydrogen and liquid oxygen both exist. Hydrogen and oxygen atoms are attracted. What Happens When You Mix Oxygen And Hydrogen.
From www.ingridscience.ca
Rocket chemistry molecular modelling (liquid oxygen/liquid hydrogen What Happens When You Mix Oxygen And Hydrogen It’s also a combustion reaction. The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. Hydrogen and oxygen arent both gasses, they are both elements.. What Happens When You Mix Oxygen And Hydrogen.
From www.alamy.com
electrolysis cell of water to produce hydrogen and oxygen Stock Photo What Happens When You Mix Oxygen And Hydrogen The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. But if you add some energy, say from a match, the gases will break. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. This. What Happens When You Mix Oxygen And Hydrogen.
From www.slideshare.net
Intro separate hydrogen and oxygen from water through electrolysis What Happens When You Mix Oxygen And Hydrogen This is a synthesis reaction that makes water from its elements. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. How do two gases combine to make liquid water? The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to. What Happens When You Mix Oxygen And Hydrogen.
From www.ingridscience.ca
Hydrogen peroxide chemistry ingridscience.ca What Happens When You Mix Oxygen And Hydrogen The balanced equation for the chemical reaction is: Liquid hydrogen and liquid oxygen both exist. How do two gases combine to make liquid water? The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. 2 h 2 + o 2 → 2 h 2 o. Making water. What Happens When You Mix Oxygen And Hydrogen.
From www.shutterstock.com
Reaction Hydrogen Oxygen New Compounds Water เวกเตอร์สต็อก (ปลอดค่า What Happens When You Mix Oxygen And Hydrogen Hydrogen and oxygen arent both gasses, they are both elements. If you combine oxygen gas and hydrogen gas, you'll still have gas. The balanced equation for the chemical reaction is: It’s also a combustion reaction. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. 2 h 2 + o 2 → 2 h 2 o. But. What Happens When You Mix Oxygen And Hydrogen.
From www.alamy.com
Reaction of Hydrogen and Oxygen in New compounds. Water molecule that What Happens When You Mix Oxygen And Hydrogen It’s also a combustion reaction. Hydrogen and oxygen arent both gasses, they are both elements. The balanced equation for the chemical reaction is: Liquid hydrogen and liquid oxygen both exist. But if you add some energy, say from a match, the gases will break. 2 h 2 + o 2 → 2 h 2 o. This is a synthesis reaction. What Happens When You Mix Oxygen And Hydrogen.
From www.alamy.com
Reaction of Hydrogen and Oxygen to water Stock Vector Image & Art Alamy What Happens When You Mix Oxygen And Hydrogen How do two gases combine to make liquid water? But if you add some energy, say from a match, the gases will break. Liquid hydrogen and liquid oxygen both exist. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. If you combine oxygen gas and hydrogen gas, you'll still have gas. This is a synthesis reaction. What Happens When You Mix Oxygen And Hydrogen.
From saylordotorg.github.io
A Description of Matter What Happens When You Mix Oxygen And Hydrogen It’s also a combustion reaction. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. The balanced equation for the chemical reaction is: This is a synthesis reaction that makes water from its elements. If you combine oxygen gas and hydrogen gas, you'll still have gas. The reverse reaction, water to hydrogen and oxygen, has a positive. What Happens When You Mix Oxygen And Hydrogen.
From sciencing.com
What Happens When Hydrogen & Oxygen Combine? Sciencing What Happens When You Mix Oxygen And Hydrogen It’s also a combustion reaction. 2 h 2 + o 2 → 2 h 2 o. Hydrogen and oxygen arent both gasses, they are both elements. How do two gases combine to make liquid water? The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. Making water. What Happens When You Mix Oxygen And Hydrogen.
From sciencenotes.org
Making Water From Hydrogen and Oxygen What Happens When You Mix Oxygen And Hydrogen 2 h 2 + o 2 → 2 h 2 o. But if you add some energy, say from a match, the gases will break. Hydrogen and oxygen arent both gasses, they are both elements. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. It’s also a combustion reaction. Liquid hydrogen and liquid oxygen both exist.. What Happens When You Mix Oxygen And Hydrogen.
From www.vectorstock.com
Electrolysis water forming hydrogen and oxygen Vector Image What Happens When You Mix Oxygen And Hydrogen It’s also a combustion reaction. Hydrogen and oxygen arent both gasses, they are both elements. This is a synthesis reaction that makes water from its elements. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. How do two gases combine to make liquid water? 2 h 2 + o 2 → 2 h 2 o. Making. What Happens When You Mix Oxygen And Hydrogen.
From thehomeschoolscientist.com
Splitting Water Into Hydrogen and Oxygen The Homeschool Scientist What Happens When You Mix Oxygen And Hydrogen The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. If you combine oxygen gas and hydrogen gas, you'll still have gas. But if you add some energy, say from a match, the gases will break. Hydrogen and oxygen atoms are attracted by positive and negative charges. What Happens When You Mix Oxygen And Hydrogen.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW What Happens When You Mix Oxygen And Hydrogen Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. The balanced equation for the chemical reaction is: If you combine oxygen gas and hydrogen gas, you'll still have gas. 2 h 2 + o 2 → 2 h 2 o. How do two gases combine to make liquid water? This is a synthesis reaction that makes. What Happens When You Mix Oxygen And Hydrogen.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID5648526 What Happens When You Mix Oxygen And Hydrogen Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. Liquid hydrogen and liquid oxygen both exist. But if you add some energy, say from a match, the gases will break. If you combine oxygen gas and hydrogen gas, you'll still have gas. It’s also a combustion reaction.. What Happens When You Mix Oxygen And Hydrogen.
From www.dreamstime.com
Hydrogen and Oxygen Atoms Nucleus and Shells Stock Vector What Happens When You Mix Oxygen And Hydrogen This is a synthesis reaction that makes water from its elements. The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. 2 h 2 +. What Happens When You Mix Oxygen And Hydrogen.
From www.youtube.com
iW8ehhWplt1VYD4cxq_axtdYxht0eWEQSZyF5H7qtx_tN3Tf2neE_cbqnn65j What Happens When You Mix Oxygen And Hydrogen It’s also a combustion reaction. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. 2 h 2 + o 2 → 2 h 2 o. The balanced equation for the chemical reaction is: This is a synthesis reaction that makes water from its elements. Hydrogen and oxygen. What Happens When You Mix Oxygen And Hydrogen.
From concord.org
Reaction Between Hydrogen and Oxygen Molecules STEM Resource Finder What Happens When You Mix Oxygen And Hydrogen How do two gases combine to make liquid water? This is a synthesis reaction that makes water from its elements. Hydrogen and oxygen arent both gasses, they are both elements. 2 h 2 + o 2 → 2 h 2 o. If you combine oxygen gas and hydrogen gas, you'll still have gas. Liquid hydrogen and liquid oxygen both exist.. What Happens When You Mix Oxygen And Hydrogen.
From www.youtube.com
Bleach and Hydrogen Peroxide Reaction + Balanced Equation YouTube What Happens When You Mix Oxygen And Hydrogen Hydrogen and oxygen arent both gasses, they are both elements. This is a synthesis reaction that makes water from its elements. The balanced equation for the chemical reaction is: Liquid hydrogen and liquid oxygen both exist. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. 2 h 2 + o 2 → 2 h 2 o.. What Happens When You Mix Oxygen And Hydrogen.
From www.slideserve.com
PPT Rates of Reaction PowerPoint Presentation, free download ID3014435 What Happens When You Mix Oxygen And Hydrogen The balanced equation for the chemical reaction is: It’s also a combustion reaction. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. This is a synthesis reaction that makes water from its elements. If you combine oxygen gas and hydrogen gas, you'll still have gas. 2 h. What Happens When You Mix Oxygen And Hydrogen.
From www.youtube.com
What Happens When Hydrogen & Oxygen Combine? YouTube What Happens When You Mix Oxygen And Hydrogen But if you add some energy, say from a match, the gases will break. How do two gases combine to make liquid water? The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. If you combine oxygen gas and hydrogen gas, you'll still have gas. 2 h. What Happens When You Mix Oxygen And Hydrogen.
From www.youtube.com
How to Balance H2S + O2 = H2O + SO2 (Hydrogen sulfide + Oxygen gas What Happens When You Mix Oxygen And Hydrogen Liquid hydrogen and liquid oxygen both exist. 2 h 2 + o 2 → 2 h 2 o. If you combine oxygen gas and hydrogen gas, you'll still have gas. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need. What Happens When You Mix Oxygen And Hydrogen.
From www.mozaweb.com
Reaction of hydrogen with oxygen 3D scene Mozaik Digital Education What Happens When You Mix Oxygen And Hydrogen Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. Liquid hydrogen and liquid oxygen both exist. This is a synthesis reaction that makes water from its elements. The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. The balanced equation for the chemical. What Happens When You Mix Oxygen And Hydrogen.
From www.youtube.com
Oxidation and Reduction in Terms of Gain or Loss of Oxygen or Hydrogen What Happens When You Mix Oxygen And Hydrogen Liquid hydrogen and liquid oxygen both exist. 2 h 2 + o 2 → 2 h 2 o. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. If you combine oxygen gas and hydrogen gas, you'll still have gas. The balanced equation for the chemical reaction is: But if you add some energy, say from a. What Happens When You Mix Oxygen And Hydrogen.
From www.dreamstime.com
Water Molecule. Oxygen and Hydrogen Stock Vector Illustration of What Happens When You Mix Oxygen And Hydrogen Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. It’s also a combustion reaction. The reverse reaction, water to hydrogen and oxygen, has a positive gibbs free energy, so you need to add energy in the form. 2 h 2 + o 2 → 2 h 2. What Happens When You Mix Oxygen And Hydrogen.
From pemf-devices.com
Hydrogen/Oxygen Therapy (H2O2 or OxyHydrogen) What Happens When You Mix Oxygen And Hydrogen This is a synthesis reaction that makes water from its elements. The balanced equation for the chemical reaction is: Liquid hydrogen and liquid oxygen both exist. 2 h 2 + o 2 → 2 h 2 o. Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. The. What Happens When You Mix Oxygen And Hydrogen.
From mavink.com
Hydrogen Oxygen Water Equation What Happens When You Mix Oxygen And Hydrogen Making water from hydrogen and oxygen is as simple as mixing hydrogen gas and oxygen gas and adding a spark or heat. 2 h 2 + o 2 → 2 h 2 o. Hydrogen and oxygen atoms are attracted by positive and negative charges which thwarts. But if you add some energy, say from a match, the gases will break.. What Happens When You Mix Oxygen And Hydrogen.