Why Boiling Stone Is Used During Distillation Process . A simple distillation is incapable of significant purification if the boiling points of the components are too close. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. When the difference in boiling. These stones have pores inside which provide cavities both to trap air and to provide spaces. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. Distillation involves boiling the solution and then.
from www.theengineeringconcepts.com
When the difference in boiling. A simple distillation is incapable of significant purification if the boiling points of the components are too close. These stones have pores inside which provide cavities both to trap air and to provide spaces. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Distillation involves boiling the solution and then.
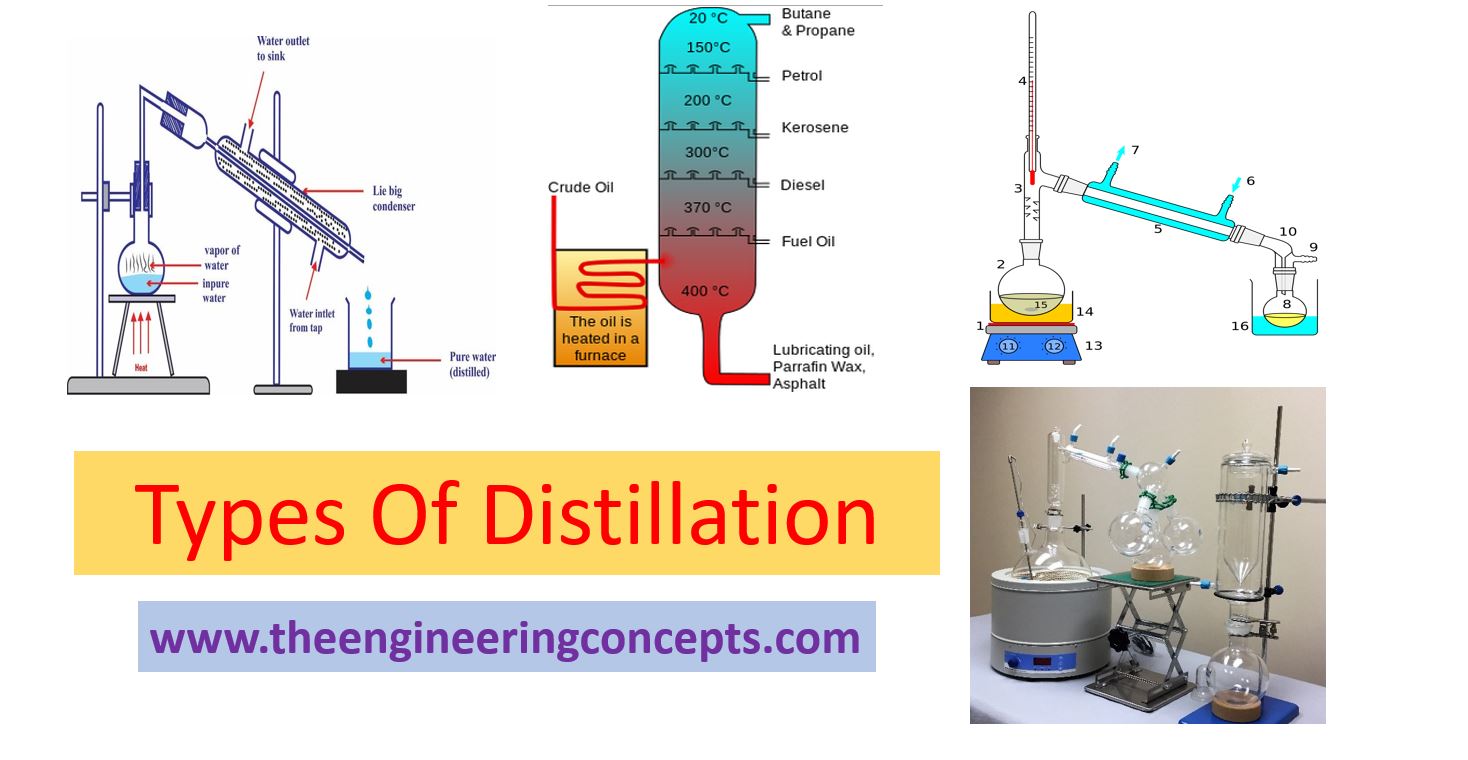
Types of Distillation The Engineering Concepts
Why Boiling Stone Is Used During Distillation Process Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Distillation involves boiling the solution and then. When the difference in boiling. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. These stones have pores inside which provide cavities both to trap air and to provide spaces. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide.
From www.thoughtco.com
What Is Distillation? Principles and Uses Why Boiling Stone Is Used During Distillation Process When the difference in boiling. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation involves boiling the solution and then. These stones have pores inside which provide cavities both to trap air and to provide spaces. Distillation columns use a thermal process that involves heating. Why Boiling Stone Is Used During Distillation Process.
From www.waterpronto.com
Water Purification By Distillation Process Explained Why Boiling Stone Is Used During Distillation Process Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. Distillation involves boiling the solution and then. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases. Why Boiling Stone Is Used During Distillation Process.
From www.youtube.com
What is Simple Distillation Separation Methods Chemistry Basics YouTube Why Boiling Stone Is Used During Distillation Process When the difference in boiling. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. Boiling chips are small, insoluble, porous stones made of calcium. Why Boiling Stone Is Used During Distillation Process.
From www.vrogue.co
Diagram Of Distillation Process Distillation Apparatu vrogue.co Why Boiling Stone Is Used During Distillation Process When the difference in boiling. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. These stones have pores inside which provide cavities both to trap air and to provide spaces. A simple distillation is incapable of. Why Boiling Stone Is Used During Distillation Process.
From mavink.com
Distillation Apparatus Labelled Diagram Why Boiling Stone Is Used During Distillation Process A simple distillation is incapable of significant purification if the boiling points of the components are too close. These stones have pores inside which provide cavities both to trap air and to provide spaces. When the difference in boiling. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. Distillation involves boiling the solution and then.. Why Boiling Stone Is Used During Distillation Process.
From zamcopter.web.fc2.com
SIMPLE DISTILLATION Why Boiling Stone Is Used During Distillation Process These stones have pores inside which provide cavities both to trap air and to provide spaces. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. Distillation columns use a thermal process that. Why Boiling Stone Is Used During Distillation Process.
From www.theengineersperspectives.com
Types of Distillation Definition, Process, Uses & Examples Why Boiling Stone Is Used During Distillation Process When the difference in boiling. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. Distillation involves boiling the solution and then. Distillation columns use a thermal process that involves heating and cooling. Why Boiling Stone Is Used During Distillation Process.
From www.vecteezy.com
Fractional distillation is a process used to separate a mixture of two Why Boiling Stone Is Used During Distillation Process Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Distillation involves boiling the solution and then. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. These stones. Why Boiling Stone Is Used During Distillation Process.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts Why Boiling Stone Is Used During Distillation Process Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. Distillation involves boiling the solution and then. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. These stones have pores inside which provide cavities both to trap air and to provide spaces. Distillation. Why Boiling Stone Is Used During Distillation Process.
From labeled-diagram.blogspot.com
Draw A Neat Labelled Diagram Of Simple Distillation Process Labeled Why Boiling Stone Is Used During Distillation Process When the difference in boiling. Distillation involves boiling the solution and then. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. These stones have. Why Boiling Stone Is Used During Distillation Process.
From www.elevise.co.uk
C1 I) Simple & Fractional Distillation AQA Combined Science Trilogy Why Boiling Stone Is Used During Distillation Process A simple distillation is incapable of significant purification if the boiling points of the components are too close. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. These stones have pores inside. Why Boiling Stone Is Used During Distillation Process.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Why Boiling Stone Is Used During Distillation Process These stones have pores inside which provide cavities both to trap air and to provide spaces. When the difference in boiling. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a. Why Boiling Stone Is Used During Distillation Process.
From www.pinterest.com
How Steam Distillation Works video by Ron Davis of chemsurvival Why Boiling Stone Is Used During Distillation Process Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation involves boiling the solution and then. These stones have pores inside which provide cavities both to trap air and to provide spaces.. Why Boiling Stone Is Used During Distillation Process.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Why Boiling Stone Is Used During Distillation Process Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. When the difference in boiling. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. A simple distillation is incapable of significant purification if the. Why Boiling Stone Is Used During Distillation Process.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Why Boiling Stone Is Used During Distillation Process Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation columns use a thermal. Why Boiling Stone Is Used During Distillation Process.
From speichim.com
Main principes of distillation Speichim Processing Valls Química Why Boiling Stone Is Used During Distillation Process When the difference in boiling. Distillation involves boiling the solution and then. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. A simple distillation. Why Boiling Stone Is Used During Distillation Process.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Why Boiling Stone Is Used During Distillation Process A simple distillation is incapable of significant purification if the boiling points of the components are too close. These stones have pores inside which provide cavities both to trap air and to provide spaces. When the difference in boiling. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent. Why Boiling Stone Is Used During Distillation Process.
From www.nagwa.com
Lesson Distillation Nagwa Why Boiling Stone Is Used During Distillation Process A simple distillation is incapable of significant purification if the boiling points of the components are too close. These stones have pores inside which provide cavities both to trap air and to provide spaces. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. When the difference in. Why Boiling Stone Is Used During Distillation Process.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Why Boiling Stone Is Used During Distillation Process When the difference in boiling. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide.. Why Boiling Stone Is Used During Distillation Process.
From www.pinterest.com
Simple Distillation GCSE / IGCSE Chemistry in 2020 Chemistry notes Why Boiling Stone Is Used During Distillation Process Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation involves boiling the solution and then. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Boiling chips are small, insoluble, porous stones made of calcium carbonate or. Why Boiling Stone Is Used During Distillation Process.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts Why Boiling Stone Is Used During Distillation Process Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation involves boiling the solution and then. A simple distillation is incapable of significant purification if the boiling. Why Boiling Stone Is Used During Distillation Process.
From www.theengineeringconcepts.com
Types of Distillation The Engineering Concepts Why Boiling Stone Is Used During Distillation Process Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation involves boiling the solution and then. These stones have pores inside which provide cavities both to trap air and to provide spaces. Distillation columns use a thermal process that involves heating and cooling to separate two. Why Boiling Stone Is Used During Distillation Process.
From www.scienceabc.com
Denatured Alcohol Definition, Properties, Examples And Uses Why Boiling Stone Is Used During Distillation Process A simple distillation is incapable of significant purification if the boiling points of the components are too close. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. These stones have pores inside which. Why Boiling Stone Is Used During Distillation Process.
From www.slideserve.com
PPT Experiment 6 Simple and Fractional Distillation PowerPoint Why Boiling Stone Is Used During Distillation Process Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. A simple distillation is incapable of significant purification if the boiling points of the components are too close.. Why Boiling Stone Is Used During Distillation Process.
From cartoondealer.com
Simple Distillation Apparatus Diagram With Full Process Vector Why Boiling Stone Is Used During Distillation Process Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. Distillation involves boiling the solution and then. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. When the difference in boiling. Distillation columns use a thermal process that involves heating and cooling to separate two or. Why Boiling Stone Is Used During Distillation Process.
From mavink.com
Labelled Diagram Of Distillation Why Boiling Stone Is Used During Distillation Process Distillation involves boiling the solution and then. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. When the difference in boiling. Distillation is a. Why Boiling Stone Is Used During Distillation Process.
From www.vecteezy.com
Distillation process diagram for education 3227893 Vector Art at Vecteezy Why Boiling Stone Is Used During Distillation Process Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. A simple distillation is incapable of significant purification if the boiling points of the components are too close. These stones have pores inside which provide cavities both to trap air and to provide spaces. Boiling chips are small,. Why Boiling Stone Is Used During Distillation Process.
From www.slideserve.com
PPT Experiment 6 Simple and Fractional Distillation PowerPoint Why Boiling Stone Is Used During Distillation Process Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. These stones have pores inside which provide cavities both to trap air and to provide spaces. Distillation involves boiling the solution and then. When the difference in boiling. Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added. Why Boiling Stone Is Used During Distillation Process.
From pharmacygyan.com
Steam distillation Principle Construction Working etc..Pharmacy Gyan Why Boiling Stone Is Used During Distillation Process Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Distillation involves boiling the solution and then. Boiling stones (or boiling chips) are small pieces of black porous rock. Why Boiling Stone Is Used During Distillation Process.
From chemistnotes.com
Distillation Definition and Types of Distillation Chemistry Notes Why Boiling Stone Is Used During Distillation Process Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. These stones have pores inside which provide cavities both to trap air and to provide spaces. A simple distillation is incapable of significant purification if the boiling points of the components are too close. When the difference in boiling. Distillation involves. Why Boiling Stone Is Used During Distillation Process.
From www.youtube.com
How To Draw Simple Distillation Diagram step by step YouTube Why Boiling Stone Is Used During Distillation Process When the difference in boiling. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. These. Why Boiling Stone Is Used During Distillation Process.
From testbook.com
team Distillation Principle, Working, Advantages & Application Why Boiling Stone Is Used During Distillation Process Boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or solution. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Distillation involves boiling the solution and then. Distillation columns use a thermal process that involves heating and cooling. Why Boiling Stone Is Used During Distillation Process.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Why Boiling Stone Is Used During Distillation Process Distillation involves boiling the solution and then. These stones have pores inside which provide cavities both to trap air and to provide spaces. Boiling chips are small, insoluble, porous stones made of calcium carbonate or silicon carbide. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Distillation columns use a thermal. Why Boiling Stone Is Used During Distillation Process.
From byjus.com
Fractional Distillation Detailed Explanation Along With Diagrams Why Boiling Stone Is Used During Distillation Process Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. These stones have pores inside which provide cavities both to trap air and to provide spaces. Distillation involves boiling the solution and then. When the difference in boiling. A simple distillation is incapable of significant purification if the. Why Boiling Stone Is Used During Distillation Process.
From slideplayer.com
Organic Chemistry Lab 315 Fall, ppt download Why Boiling Stone Is Used During Distillation Process Distillation columns use a thermal process that involves heating and cooling to separate two or more mixed substances, such as gases or. A simple distillation is incapable of significant purification if the boiling points of the components are too close. When the difference in boiling. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and. Why Boiling Stone Is Used During Distillation Process.