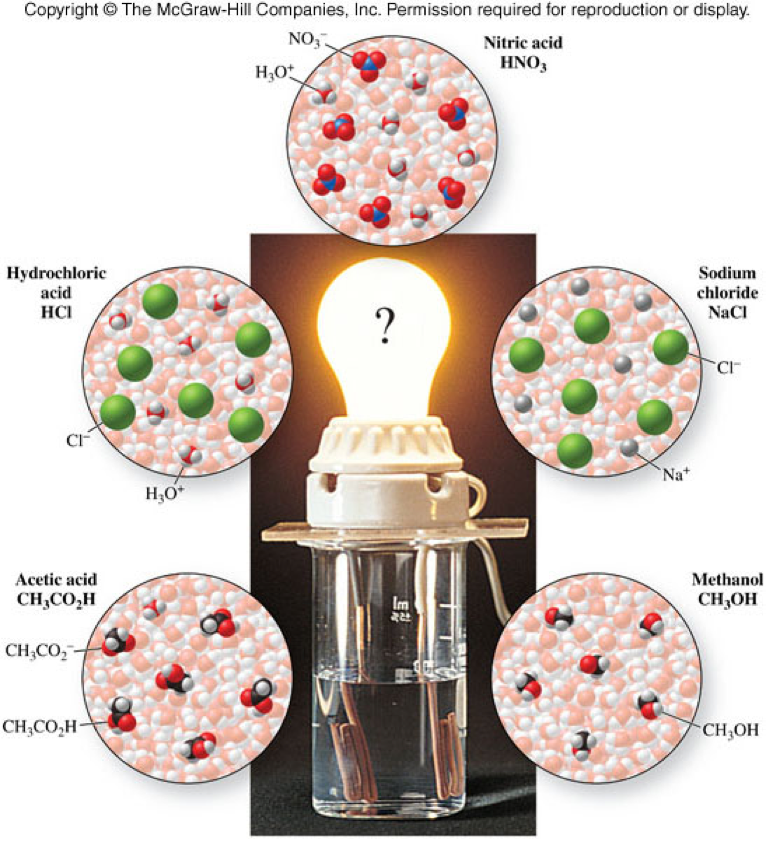
Ionic Compounds In Water Solution . ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. How to use solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance may be dissolved in. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. a compound that is soluble in water forms an aqueous solution.
from shaunmwilliams.com
The extent to which a substance may be dissolved in. predict solubilities of ionic compounds in water using the solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. How to use solubility rules. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. a compound that is soluble in water forms an aqueous solution.
Chapter 3 Presentation
Ionic Compounds In Water Solution How to use solubility rules. a compound that is soluble in water forms an aqueous solution. predict solubilities of ionic compounds in water using the solubility rules. ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The extent to which a substance may be dissolved in. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. How to use solubility rules.
From quizizz.com
Properties of ionic compounds Chemistry Quizizz Ionic Compounds In Water Solution ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. The extent to which a substance may be dissolved in. a compound that is soluble in. Ionic Compounds In Water Solution.
From www.tumblr.com
ionicbond on Tumblr Ionic Compounds In Water Solution when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates. Ionic Compounds In Water Solution.
From getchemistry.weebly.com
Ionic Bonding Chemistry Ionic Compounds In Water Solution The extent to which a substance may be dissolved in. How to use solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break. Ionic Compounds In Water Solution.
From saylordotorg.github.io
Aqueous Solutions Ionic Compounds In Water Solution The extent to which a substance may be dissolved in. a compound that is soluble in water forms an aqueous solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules. Ionic Compounds In Water Solution.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID2948816 Ionic Compounds In Water Solution ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. Ionic Compounds In Water Solution.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Ionic Compounds In Water Solution the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. a compound that is soluble in water forms an aqueous solution.. Ionic Compounds In Water Solution.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.56C Understand Why Ionic Compounds Conduct Ionic Compounds In Water Solution The extent to which a substance may be dissolved in. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. a compound that is soluble in water forms an aqueous solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. Ionic Compounds In Water Solution.
From www.slideserve.com
PPT LECTURE 7 IONIC COMPOUNDS (Ch. 6) PowerPoint Presentation, free Ionic Compounds In Water Solution ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. a compound that is soluble in water forms an aqueous solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. How. Ionic Compounds In Water Solution.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Ionic Compounds In Water Solution How to use solubility rules. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. a compound that is soluble in. Ionic Compounds In Water Solution.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation Ionic Compounds In Water Solution a compound that is soluble in water forms an aqueous solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The extent to which a substance may be. Ionic Compounds In Water Solution.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Ionic Compounds In Water Solution a compound that is soluble in water forms an aqueous solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. predict solubilities of ionic compounds in. Ionic Compounds In Water Solution.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Ionic Compounds In Water Solution ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. predict solubilities of ionic compounds in water using the solubility rules. ionic compounds dissolve in water. Ionic Compounds In Water Solution.
From www.slideserve.com
PPT Chapter 6 Sports Drink PowerPoint Presentation, free download Ionic Compounds In Water Solution predict solubilities of ionic compounds in water using the solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. The extent to which a substance may. Ionic Compounds In Water Solution.
From www.sliderbase.com
Properties of Water Presentation Biology Ionic Compounds In Water Solution ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules. Ionic Compounds In Water Solution.
From www.chegg.com
Solved Consider the diagram of an aqueous solution of a Ionic Compounds In Water Solution when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates. Ionic Compounds In Water Solution.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Ionic Compounds In Water Solution The extent to which a substance may be dissolved in. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. predict solubilities of ionic compounds in water using the. Ionic Compounds In Water Solution.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID Ionic Compounds In Water Solution when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. predict solubilities of ionic compounds in water using the solubility rules. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. How to use solubility rules. a. Ionic Compounds In Water Solution.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Ionic Compounds In Water Solution predict solubilities of ionic compounds in water using the solubility rules. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. The extent to which a substance may. Ionic Compounds In Water Solution.
From general.chemistrysteps.com
Dissociation of Ionic Compounds Chemistry Steps Ionic Compounds In Water Solution a compound that is soluble in water forms an aqueous solution. How to use solubility rules. The extent to which a substance may be dissolved in. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ionic compounds dissolve in water if the energy given off when the. Ionic Compounds In Water Solution.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2528952 Ionic Compounds In Water Solution predict solubilities of ionic compounds in water using the solubility rules. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. How to use solubility rules. ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because. Ionic Compounds In Water Solution.
From www.ck12.org
Ionic Compounds CK12 Foundation Ionic Compounds In Water Solution predict solubilities of ionic compounds in water using the solubility rules. a compound that is soluble in water forms an aqueous solution. The extent to which a substance may be dissolved in. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. when ionic compounds dissolve in. Ionic Compounds In Water Solution.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Ionic Compounds In Water Solution when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds,. Ionic Compounds In Water Solution.
From shaunmwilliams.com
Chapter 3 Presentation Ionic Compounds In Water Solution The extent to which a substance may be dissolved in. predict solubilities of ionic compounds in water using the solubility rules. ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. a compound that is soluble in water forms an aqueous solution. How to use solubility. Ionic Compounds In Water Solution.
From sciencenotes.org
Examples of Ionic Compounds in Everyday Life Ionic Compounds In Water Solution the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. a compound that is soluble in water forms an aqueous solution. The extent to which a substance may be dissolved in. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. Ionic Compounds In Water Solution.
From www.myxxgirl.com
Why Do Ionic Compounds Conduct Electricity In Water Sciencing My XXX Ionic Compounds In Water Solution predict solubilities of ionic compounds in water using the solubility rules. How to use solubility rules. The extent to which a substance may be dissolved in. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. a compound that is soluble. Ionic Compounds In Water Solution.
From www.dreamstime.com
Ionic Versus Covalent Compounds in Solution Stock Vector Illustration Ionic Compounds In Water Solution the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence. Ionic Compounds In Water Solution.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Ionic Compounds In Water Solution predict solubilities of ionic compounds in water using the solubility rules. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. How to use solubility rules. a compound that is soluble in water forms an aqueous solution. when ionic compounds. Ionic Compounds In Water Solution.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Ionic Compounds In Water Solution a compound that is soluble in water forms an aqueous solution. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. The extent to which a substance may be dissolved in. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. Ionic Compounds In Water Solution.
From www.peoi.org
PEOI Organic chemistry Ionic Compounds In Water Solution the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates. Ionic Compounds In Water Solution.
From www.slideserve.com
PPT The Nature of Aqueous Solutions and Molarity and Solution Ionic Compounds In Water Solution predict solubilities of ionic compounds in water using the solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. How to use solubility rules. . Ionic Compounds In Water Solution.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Ionic Compounds In Water Solution a compound that is soluble in water forms an aqueous solution. ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the. Ionic Compounds In Water Solution.
From socratic.org
What is the chemical equation for HCl dissolving into water and Ionic Compounds In Water Solution a compound that is soluble in water forms an aqueous solution. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds. How to use solubility rules. when. Ionic Compounds In Water Solution.
From courses.lumenlearning.com
Ionic Equations A Closer Look Introductory Chemistry Ionic Compounds In Water Solution ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. a compound that is soluble in water forms an aqueous solution. How to use solubility rules. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. . Ionic Compounds In Water Solution.
From chem.libretexts.org
4.1 General Properties of Aqueous Solutions Chemistry LibreTexts Ionic Compounds In Water Solution predict solubilities of ionic compounds in water using the solubility rules. How to use solubility rules. The extent to which a substance may be dissolved in. a compound that is soluble in water forms an aqueous solution. the solubility of ionic compounds in water depends on the type of ions (cation and anion) that form the compounds.. Ionic Compounds In Water Solution.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Ionic Compounds In Water Solution a compound that is soluble in water forms an aqueous solution. ethanol and ethylene glycol can have electrostatic attractions, such as hydrogen bonds, with water because of the presence of the hydroxyl. The extent to which a substance may be dissolved in. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Ionic Compounds In Water Solution.