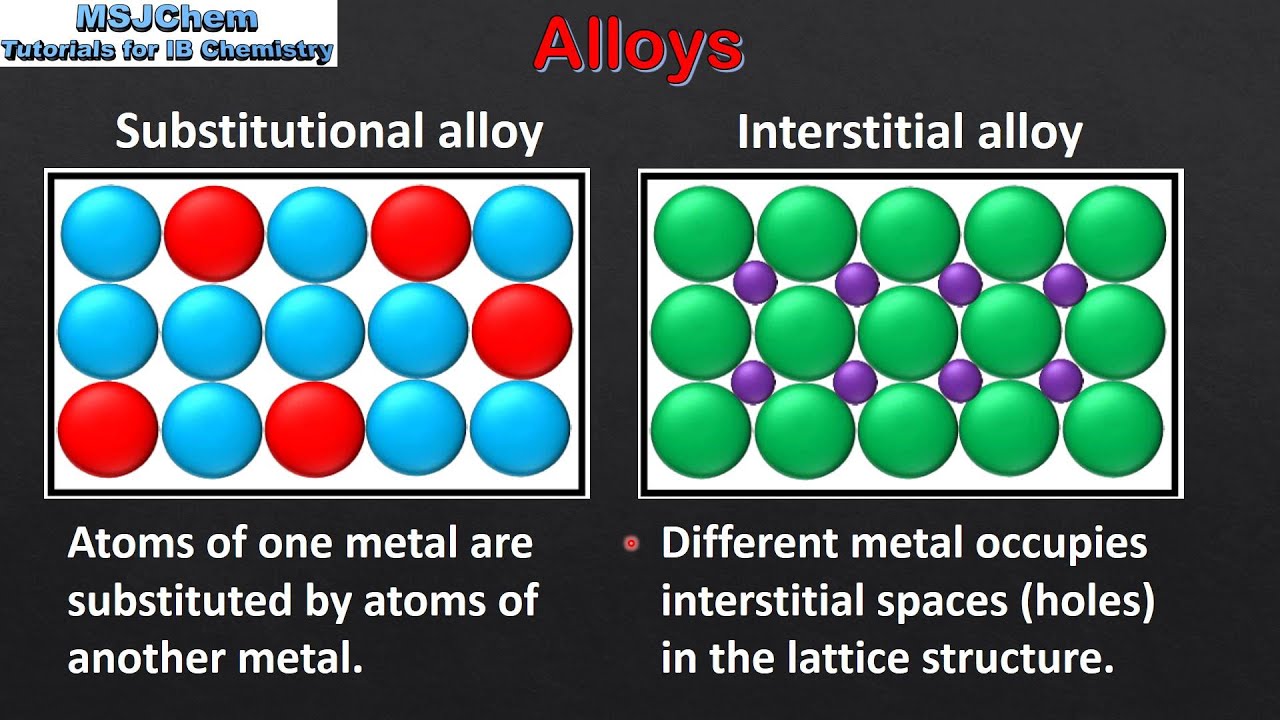
Structure Of Metals And Alloys . the structure of metals explains their high melting and boiling points and their conductivity. Metallic bonding is strong meaning that most metals have very high melting and boiling. The structure of metals explains their high melting and boiling points and their conductivity. The properties of a metal. structure and properties of metals. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. most metals and alloys crystallize in one of three very common structures: the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common alloys.
from www.animalia-life.club
The structure of metals explains their high melting and boiling points and their conductivity. The properties of a metal. the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common alloys. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. Metallic bonding is strong meaning that most metals have very high melting and boiling. most metals and alloys crystallize in one of three very common structures: structure and properties of metals. the structure of metals explains their high melting and boiling points and their conductivity. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys.
Alloys Chemistry
Structure Of Metals And Alloys The properties of a metal. The properties of a metal. most metals and alloys crystallize in one of three very common structures: Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common alloys. Metallic bonding is strong meaning that most metals have very high melting and boiling. the structure of metals explains their high melting and boiling points and their conductivity. The structure of metals explains their high melting and boiling points and their conductivity. structure and properties of metals. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys.
From www.youtube.com
Uses of Metals and their Alloys GCSE Separate Chemistry YouTube Structure Of Metals And Alloys The properties of a metal. the structure of metals explains their high melting and boiling points and their conductivity. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common. Structure Of Metals And Alloys.
From www.dierk-raabe.com
Metallurgical Materials Science and Alloy Design Aluminium alloys Structure Of Metals And Alloys The properties of a metal. The structure of metals explains their high melting and boiling points and their conductivity. most metals and alloys crystallize in one of three very common structures: Metallic bonding is strong meaning that most metals have very high melting and boiling. the structure of metals explains their high melting and boiling points and their. Structure Of Metals And Alloys.
From schematicfixgingles.z21.web.core.windows.net
Phase Diagram Of Metals Structure Of Metals And Alloys there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. The structure of metals explains their high melting and boiling points and their conductivity. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. most metals and alloys crystallize in one of three very common structures: Metallic bonding is. Structure Of Metals And Alloys.
From www.iqsdirectory.com
Metal Alloys Principles, Types, Advantages and Applications Structure Of Metals And Alloys the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common alloys. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. structure and properties of metals. The structure of metals explains their high melting and boiling points and their conductivity. . Structure Of Metals And Alloys.
From guidedbconmonoplegia.z13.web.core.windows.net
Fec Phase Diagram Microstructure Structure Of Metals And Alloys structure and properties of metals. The structure of metals explains their high melting and boiling points and their conductivity. most metals and alloys crystallize in one of three very common structures: the structure of metals explains their high melting and boiling points and their conductivity. The properties of a metal. the structure of the metal alloy. Structure Of Metals And Alloys.
From studykaki.com
Explain, using structure and bonding, why alloys are stronger and Structure Of Metals And Alloys Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. Metallic bonding is strong meaning that most metals have very high melting and boiling. structure and properties of metals. the structure of metals explains their high melting and boiling points and their conductivity. the structure of the metal alloy is not limited to. Structure Of Metals And Alloys.
From chemistrylabs-2.blogspot.com
Why Are Alloys Harder Than Pure Metals Chemistry Labs Structure Of Metals And Alloys The properties of a metal. the structure of metals explains their high melting and boiling points and their conductivity. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. The structure of metals explains their high melting and boiling. Structure Of Metals And Alloys.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID3827489 Structure Of Metals And Alloys Metallic bonding is strong meaning that most metals have very high melting and boiling. the structure of metals explains their high melting and boiling points and their conductivity. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. most metals and alloys crystallize in one of three very common structures: Identify and. Structure Of Metals And Alloys.
From www.mdpi.com
Metals Free FullText A Review of Microstructural Evolution and Structure Of Metals And Alloys The properties of a metal. Metallic bonding is strong meaning that most metals have very high melting and boiling. the structure of metals explains their high melting and boiling points and their conductivity. structure and properties of metals. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. the structure of the metal. Structure Of Metals And Alloys.
From www.youtube.com
2.4 Structure of Metals and Alloys YouTube Structure Of Metals And Alloys structure and properties of metals. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. The structure of metals explains their high melting and boiling points and their conductivity. Metallic bonding is strong meaning that most metals have very high melting and boiling. The properties of a metal. the structure of metals explains their. Structure Of Metals And Alloys.
From sciencenotes.org
What Is an Alloy? Definition and Examples Structure Of Metals And Alloys most metals and alloys crystallize in one of three very common structures: the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common alloys. The properties of a metal. the structure of metals explains their high melting and boiling points and their conductivity. there. Structure Of Metals And Alloys.
From news.mit.edu
Researchers find a better way to design metal alloys MIT News Structure Of Metals And Alloys most metals and alloys crystallize in one of three very common structures: The structure of metals explains their high melting and boiling points and their conductivity. The properties of a metal. Metallic bonding is strong meaning that most metals have very high melting and boiling. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within.. Structure Of Metals And Alloys.
From www.dreamstime.com
Alloy Vs Pure Metal Comparison with Iron and Steel Properties Outline Structure Of Metals And Alloys structure and properties of metals. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. The structure of metals explains their high melting and boiling points and their conductivity. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. the structure of metals explains their high melting and. Structure Of Metals And Alloys.
From en.ppt-online.org
The structure of Metals online presentation Structure Of Metals And Alloys there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. the structure of metals explains their high melting and boiling points and their conductivity. The properties of a metal. The structure of metals explains their high melting and boiling points and their conductivity. Identify and assign unit cells, coordination numbers, asymmetric units, numbers. Structure Of Metals And Alloys.
From matsciwit.blogspot.com
Materials Witness The Elemental Composition of Metal Alloys Structure Of Metals And Alloys Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. structure and properties of metals. Metallic bonding is strong meaning that most metals have very high melting and boiling. The structure of metals explains their high melting and boiling points and their conductivity. The properties of a metal. there are roughly 70 metallic and. Structure Of Metals And Alloys.
From www.tec-science.com
Typs of alloys tecscience Structure Of Metals And Alloys The structure of metals explains their high melting and boiling points and their conductivity. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. the structure of metals explains their high melting and boiling points and their conductivity. most metals and alloys crystallize in one of three very common structures: structure and properties. Structure Of Metals And Alloys.
From www.alamy.com
Aluminium (aluminum) metal, crystal structure Stock Photo Alamy Structure Of Metals And Alloys the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common alloys. structure and properties of metals. the structure of metals explains their high melting and boiling points and their conductivity. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations. Structure Of Metals And Alloys.
From msestudent.com
The Difference Between Alloys and Composites (and Compounds Structure Of Metals And Alloys Metallic bonding is strong meaning that most metals have very high melting and boiling. The properties of a metal. The structure of metals explains their high melting and boiling points and their conductivity. most metals and alloys crystallize in one of three very common structures: the structure of the metal alloy is not limited to these two structures,. Structure Of Metals And Alloys.
From app.pandai.org
Alloy Structure Of Metals And Alloys there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. the structure of metals explains their high melting and boiling points and their conductivity. most metals and alloys crystallize in one of three very common structures: structure and properties of metals. the structure of the metal alloy is not limited. Structure Of Metals And Alloys.
From studentlesson.com
Common types of metals, their properties and uses student lesson Structure Of Metals And Alloys Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. Metallic bonding is strong meaning that most metals have very high melting and boiling. the structure of metals explains their high melting and boiling points and their conductivity. The. Structure Of Metals And Alloys.
From dxofkurut.blob.core.windows.net
Alloy Steel Definition Example at Matthew Reid blog Structure Of Metals And Alloys most metals and alloys crystallize in one of three very common structures: The structure of metals explains their high melting and boiling points and their conductivity. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. structure and properties of metals. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of. Structure Of Metals And Alloys.
From msestudent.com
The Difference Between Alloys and Composites (and Compounds Structure Of Metals And Alloys The structure of metals explains their high melting and boiling points and their conductivity. structure and properties of metals. The properties of a metal. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. Metallic bonding is strong meaning that most metals have very high melting and boiling. the structure of the. Structure Of Metals And Alloys.
From lakewilber54beau.water.blog
Steel Or Metal Structures What Is Best? The Life of Banke 575 Structure Of Metals And Alloys most metals and alloys crystallize in one of three very common structures: there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. the structure of metals explains their high melting and boiling points and their conductivity. the structure of the metal alloy is not limited to these two structures, but combined. Structure Of Metals And Alloys.
From studylib.net
2.4 Structure of Metals and Alloys Structure Of Metals And Alloys there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common alloys. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. Metallic bonding is strong meaning. Structure Of Metals And Alloys.
From kieran-jolpblogcooke.blogspot.com
Explain the Differences in Properties Between Pure Metals and Alloys Structure Of Metals And Alloys Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. The properties of a metal. the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common alloys.. Structure Of Metals And Alloys.
From www.animalia-life.club
Alloys Chemistry Structure Of Metals And Alloys Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common alloys. The structure of metals explains their high melting and boiling points and their conductivity. structure and properties of metals. The. Structure Of Metals And Alloys.
From tampasteel.com
What Is a Metal Alloy? Metal vs Metal Alloy Difference Tampa Steel Structure Of Metals And Alloys the structure of metals explains their high melting and boiling points and their conductivity. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. Metallic bonding is strong meaning that most metals have very high melting and boiling. The structure of metals explains their high melting and boiling points and their conductivity. . Structure Of Metals And Alloys.
From www.slideserve.com
PPT Solids PowerPoint Presentation, free download ID2735393 Structure Of Metals And Alloys The properties of a metal. the structure of metals explains their high melting and boiling points and their conductivity. structure and properties of metals. Metallic bonding is strong meaning that most metals have very high melting and boiling. the structure of the metal alloy is not limited to these two structures, but combined they represent a large. Structure Of Metals And Alloys.
From www.thoughtco.com
Properties, Composition, and Production of Metal Alloys Structure Of Metals And Alloys The structure of metals explains their high melting and boiling points and their conductivity. the structure of metals explains their high melting and boiling points and their conductivity. The properties of a metal. structure and properties of metals. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. the structure of. Structure Of Metals And Alloys.
From thechemistrynotes.com
Structure of Metals and Alloys Atomic and Crystal Structure Structure Of Metals And Alloys The structure of metals explains their high melting and boiling points and their conductivity. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. Metallic bonding is strong meaning that most metals have very high melting and boiling. structure and properties of metals. the structure of the metal alloy is not limited to these. Structure Of Metals And Alloys.
From www.wasatchsteel.com
All You Need to Know About Alloys, Part 2 Wasatch Steel Structure Of Metals And Alloys The properties of a metal. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common alloys. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within.. Structure Of Metals And Alloys.
From msestudent.com
The Difference Between Alloys and Composites (and Compounds Structure Of Metals And Alloys The properties of a metal. most metals and alloys crystallize in one of three very common structures: structure and properties of metals. The structure of metals explains their high melting and boiling points and their conductivity. the structure of metals explains their high melting and boiling points and their conductivity. Metallic bonding is strong meaning that most. Structure Of Metals And Alloys.
From ar.inspiredpencil.com
Metal Crystal Structure Structure Of Metals And Alloys there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. The structure of metals explains their high melting and boiling points and their conductivity. structure and properties of metals. most metals and alloys crystallize in one of three. Structure Of Metals And Alloys.
From cronodon.com
Metals Structure Of Metals And Alloys The structure of metals explains their high melting and boiling points and their conductivity. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. structure and properties of metals. the structure of the metal alloy is not limited to these two structures, but combined they represent a large portion of the common. Structure Of Metals And Alloys.
From www.slideserve.com
PPT Solids PowerPoint Presentation, free download ID2735393 Structure Of Metals And Alloys The properties of a metal. structure and properties of metals. Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within. there are roughly 70 metallic and metallike elements with correspondingly 2,485 binary combinations or alloys. The structure of metals explains their high melting and boiling points and their conductivity. Metallic bonding is strong meaning. Structure Of Metals And Alloys.