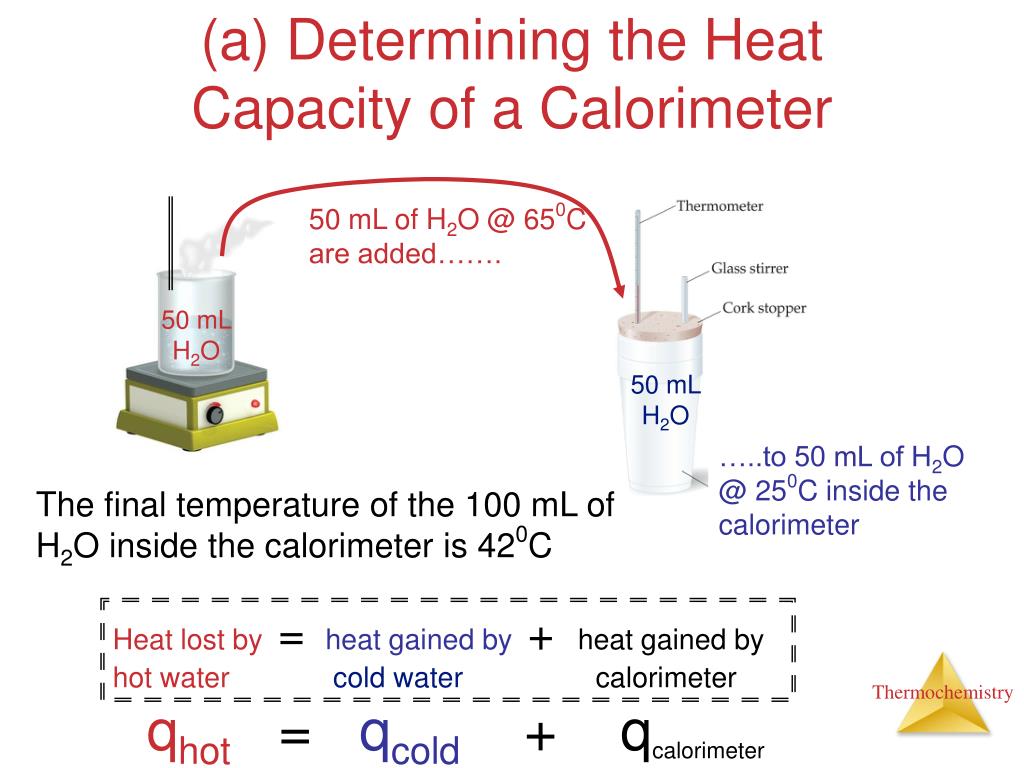
How To Find Heat Capacity Of Calorimeter With Hot And Cold Water . express the heat gained by the water in terms of the mass of the water, the specific heat of water, the initial temperature of the water, and the final temperature: heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from the hot to f (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). Then, it absorbs heat until all of it melts. Calculate and interpret heat and related properties. is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. calculate the heat capacity of the calorimeter. first, the ice absorbs heat until it reaches 0 degrees. Lastly, it absorbs heat until it reaches 20 c. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. the simplest way to experimentally find the heat capacity of a calorimeter is to mix equal quantities of hot and cold water in the calorimeter. Explain the technique of calorimetry.
from www.slideserve.com
Explain the technique of calorimetry. calculate the heat capacity of the calorimeter. Then, it absorbs heat until all of it melts. Lastly, it absorbs heat until it reaches 20 c. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. first, the ice absorbs heat until it reaches 0 degrees. the simplest way to experimentally find the heat capacity of a calorimeter is to mix equal quantities of hot and cold water in the calorimeter. heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from the hot to f (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). express the heat gained by the water in terms of the mass of the water, the specific heat of water, the initial temperature of the water, and the final temperature:
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download
How To Find Heat Capacity Of Calorimeter With Hot And Cold Water heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from the hot to f heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from the hot to f Lastly, it absorbs heat until it reaches 20 c. is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). express the heat gained by the water in terms of the mass of the water, the specific heat of water, the initial temperature of the water, and the final temperature: first, the ice absorbs heat until it reaches 0 degrees. Then, it absorbs heat until all of it melts. calculate the heat capacity of the calorimeter. Explain the technique of calorimetry. Calculate and interpret heat and related properties. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. the simplest way to experimentally find the heat capacity of a calorimeter is to mix equal quantities of hot and cold water in the calorimeter.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download How To Find Heat Capacity Of Calorimeter With Hot And Cold Water (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). Explain the technique of calorimetry. heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Lastly, it absorbs heat until it reaches 20 c. Calculate and interpret heat and related properties. calculate the heat capacity of the calorimeter. is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. Explain the technique of. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper How To Find Heat Capacity Of Calorimeter With Hot And Cold Water (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Calculate. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Then, it absorbs heat until all of it melts. first, the ice absorbs heat until it reaches 0 degrees. express the heat gained by the water in terms of the mass of the water, the specific heat of water, the initial temperature of the water, and the final temperature: is the heat capacity of the hot water,. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.youtube.com
How to find calorimeter constant YouTube How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Lastly, it absorbs heat until it reaches 20 c. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Calculate and interpret heat and related properties. Then, it absorbs heat until all of it melts. the simplest way to experimentally find the heat capacity of a calorimeter is to mix equal quantities of hot and. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry How To Find Heat Capacity Of Calorimeter With Hot And Cold Water is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. first, the ice absorbs heat until it reaches 0 degrees. heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry How To Find Heat Capacity Of Calorimeter With Hot And Cold Water calculate the heat capacity of the calorimeter. Lastly, it absorbs heat until it reaches 20 c. (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). Calculate and interpret heat and related properties. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Then, it absorbs heat until all of it melts.. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube How To Find Heat Capacity Of Calorimeter With Hot And Cold Water is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. Lastly, it absorbs heat until it reaches 20 c. first, the ice absorbs heat until it reaches 0 degrees. the combustion of 1 mole of glucose. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Explain the technique of calorimetry. (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). the simplest way to experimentally find the heat capacity of a calorimeter is to mix equal quantities of hot and cold water in the calorimeter. calculate the heat capacity of the calorimeter. Then, it absorbs heat until all of it. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Explain the technique of calorimetry. Lastly, it absorbs heat until it reaches 20 c. (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). express the heat gained by the water in terms of the mass of the water, the specific heat of water, the initial temperature of the water, and the final temperature: calculate. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How To Find Heat Capacity Of Calorimeter With Hot And Cold Water first, the ice absorbs heat until it reaches 0 degrees. (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. the simplest way. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Explain the technique of calorimetry. heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from the hot to f Then, it absorbs heat until all of it melts.. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog How To Find Heat Capacity Of Calorimeter With Hot And Cold Water heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from the hot to f Lastly, it absorbs heat until it reaches 20 c. (the specific heat capacity of. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Calculate and interpret heat and related properties. Explain the technique of calorimetry. Lastly, it absorbs heat until it reaches 20 c. Then, it absorbs heat until all of it melts. the simplest way to experimentally find the heat capacity of a calorimeter is to mix equal quantities of hot and cold water in the calorimeter. first, the ice. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Then, it absorbs heat until all of it melts. first, the ice absorbs heat until it reaches 0 degrees. heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 How To Find Heat Capacity Of Calorimeter With Hot And Cold Water the simplest way to experimentally find the heat capacity of a calorimeter is to mix equal quantities of hot and cold water in the calorimeter. is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. calculate. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer How To Find Heat Capacity Of Calorimeter With Hot And Cold Water first, the ice absorbs heat until it reaches 0 degrees. (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). express the heat gained by the water in terms of the mass of the water, the specific heat of water, the initial temperature of the water, and the final temperature: the simplest way to. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Explain the technique of calorimetry. Calculate and interpret heat and related properties. Then, it absorbs heat until all of it melts. is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. express the heat gained by the. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How To Find Heat Capacity Of Calorimeter With Hot And Cold Water heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from the hot to f express the heat gained by the water in terms of the mass of. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.animalia-life.club
Calorimeter Diagram How To Find Heat Capacity Of Calorimeter With Hot And Cold Water heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from the hot to f express the heat gained by the water in terms of the mass of. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle How To Find Heat Capacity Of Calorimeter With Hot And Cold Water the simplest way to experimentally find the heat capacity of a calorimeter is to mix equal quantities of hot and cold water in the calorimeter. Calculate and interpret heat and related properties. calculate the heat capacity of the calorimeter. is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From exospxjed.blob.core.windows.net
Calorimeter Constant at Wayne Mike blog How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Calculate and interpret heat and related properties. Explain the technique of calorimetry. first, the ice absorbs heat until it reaches 0 degrees. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. calculate the heat capacity of the calorimeter. express the heat gained by the water in terms of the mass of the. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Calculate and interpret heat and related properties. Lastly, it absorbs heat until it reaches 20 c. heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from the hot. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Explain the technique of calorimetry. Then, it absorbs heat until all of it melts. Calculate and interpret heat and related properties. first, the ice absorbs heat until it reaches 0 degrees. heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.chegg.com
Solved Calculate Heat capacity of the calorimeter (J/ ºC) , How To Find Heat Capacity Of Calorimeter With Hot And Cold Water is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. Lastly, it absorbs heat until it reaches 20 c. (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). heat transfer between hot and. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From exozekuiy.blob.core.windows.net
Junkers Calorimeter Working at Ronny Harbor blog How To Find Heat Capacity Of Calorimeter With Hot And Cold Water (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). Calculate and interpret heat and related properties. heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog How To Find Heat Capacity Of Calorimeter With Hot And Cold Water the simplest way to experimentally find the heat capacity of a calorimeter is to mix equal quantities of hot and cold water in the calorimeter. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From users.highland.edu
Calorimetry How To Find Heat Capacity Of Calorimeter With Hot And Cold Water calculate the heat capacity of the calorimeter. (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii How To Find Heat Capacity Of Calorimeter With Hot And Cold Water the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. express the heat gained by the water in terms of the mass of. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.numerade.com
SOLVEDCHEMISTRY DETERMINING HEAT CAPACITY OF A CALORIMETER How To Find Heat Capacity Of Calorimeter With Hot And Cold Water calculate the heat capacity of the calorimeter. Lastly, it absorbs heat until it reaches 20 c. (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer How To Find Heat Capacity Of Calorimeter With Hot And Cold Water express the heat gained by the water in terms of the mass of the water, the specific heat of water, the initial temperature of the water, and the final temperature: the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Lastly, it absorbs heat until it reaches 20 c. is the heat capacity of. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube How To Find Heat Capacity Of Calorimeter With Hot And Cold Water (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). the simplest way to experimentally find the heat capacity of a calorimeter is to mix equal quantities of hot and cold water in the calorimeter. Explain the technique of calorimetry. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. first,. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation How To Find Heat Capacity Of Calorimeter With Hot And Cold Water heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c) energy is transferred between the two, but the rate of heat transfer from the hot to f Calculate and interpret heat and related properties. express the heat gained by the. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.slideshare.net
Tang 01 heat capacity and calorimetry How To Find Heat Capacity Of Calorimeter With Hot And Cold Water (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). first, the ice absorbs heat until it reaches 0 degrees. is the heat capacity of the hot water, ccw is the heat capacity of the cold water, δthw is the temperature change experienced by the hot water as a result of. express the heat. How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.
From www.youtube.com
Specific heat capacity YouTube How To Find Heat Capacity Of Calorimeter With Hot And Cold Water Calculate and interpret heat and related properties. (the specific heat capacity of water is 4.184 j g¯ 1 c¯ 1). first, the ice absorbs heat until it reaches 0 degrees. heat transfer between hot and cold objects in an ideal calorimeter when a hot object (t h) is placed in thermal contact with a cold object (t c). How To Find Heat Capacity Of Calorimeter With Hot And Cold Water.