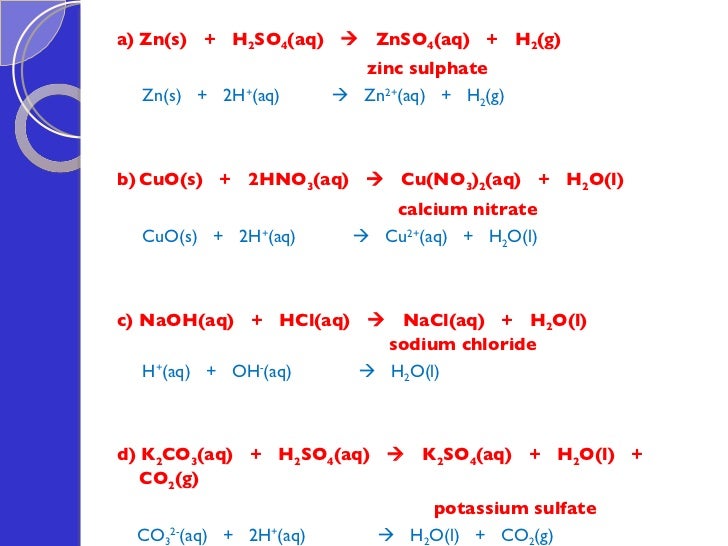
Zinc And Hydrochloric Acid Equation Balanced . The experiment compares reactivity of three. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Look here for some awesome experiments with zinc. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: Zinc sulfate [wikimedia] zinc is a heavy metal compared with lithium, for instance, so the bubbles don’t rise to the surface. enter an equation of an ionic chemical equation and press the balance button. this experiment includes the reaction of zinc with hydrochloric acid. in this video, we'll be exploring the reaction between zinc metal and. zinc metal reacts with hydrochloric acid according to the balanced equation: an explanation of how to balance and write the the equation for zinc. H₂so₄ + zn = znso₄ + h₂↑. zn + 2h₂o = zno + h₂↑. The balanced equation will be calculated along. this chemistry video tutorial explains how to predict the products of the.
from www.slideshare.net
Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. enter an equation of an ionic chemical equation and press the balance button. Look here for some awesome experiments with zinc. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. this chemistry video tutorial explains how to predict the products of the. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: Zinc sulfate [wikimedia] zinc is a heavy metal compared with lithium, for instance, so the bubbles don’t rise to the surface. H₂so₄ + zn = znso₄ + h₂↑. zinc metal reacts with hydrochloric acid according to the balanced equation: zn + 2h₂o = zno + h₂↑.
Acids And Bases
Zinc And Hydrochloric Acid Equation Balanced this experiment includes the reaction of zinc with hydrochloric acid. Zinc sulfate [wikimedia] zinc is a heavy metal compared with lithium, for instance, so the bubbles don’t rise to the surface. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Look here for some awesome experiments with zinc. this chemistry video tutorial explains how to predict the products of the. zn + 2h₂o = zno + h₂↑. The balanced equation will be calculated along. zinc metal reacts with hydrochloric acid according to the balanced equation: Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. in this video, we'll be exploring the reaction between zinc metal and. enter an equation of an ionic chemical equation and press the balance button. an explanation of how to balance and write the the equation for zinc. The experiment compares reactivity of three. this experiment includes the reaction of zinc with hydrochloric acid. H₂so₄ + zn = znso₄ + h₂↑. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released:
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc And Hydrochloric Acid Equation Balanced Look here for some awesome experiments with zinc. zinc metal reacts with hydrochloric acid according to the balanced equation: zn + 2h₂o = zno + h₂↑. this experiment includes the reaction of zinc with hydrochloric acid. this chemistry video tutorial explains how to predict the products of the. zn + hcl = zncl2 + h2. Zinc And Hydrochloric Acid Equation Balanced.
From dxorwmpbw.blob.core.windows.net
Word Equation Between Zinc And Hydrochloric Acid at Noah Brooks blog Zinc And Hydrochloric Acid Equation Balanced in this video, we'll be exploring the reaction between zinc metal and. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. The experiment compares reactivity of three. enter an equation of an ionic chemical equation and press the balance button. Zn (s) +. Zinc And Hydrochloric Acid Equation Balanced.
From byjus.com
Zinc and hydrochloric acid react according t reaction Zn(s) + 2HCI(aq Zinc And Hydrochloric Acid Equation Balanced zinc metal reacts with hydrochloric acid according to the balanced equation: The experiment compares reactivity of three. zn + 2h₂o = zno + h₂↑. H₂so₄ + zn = znso₄ + h₂↑. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. zn + hcl = zncl2 +. Zinc And Hydrochloric Acid Equation Balanced.
From www.quanswer.com
Write the balanced equation for the reaction of zinc oxide with Zinc And Hydrochloric Acid Equation Balanced zn + 2h₂o = zno + h₂↑. Zinc sulfate [wikimedia] zinc is a heavy metal compared with lithium, for instance, so the bubbles don’t rise to the surface. The balanced equation will be calculated along. Look here for some awesome experiments with zinc. H₂so₄ + zn = znso₄ + h₂↑. in this video, we'll be exploring the reaction. Zinc And Hydrochloric Acid Equation Balanced.
From www.youtube.com
How to Balance Zn(OH)2 + HCl = ZnCl2 + H2O (Zinc Hydroxide Zinc And Hydrochloric Acid Equation Balanced an explanation of how to balance and write the the equation for zinc. zn + 2h₂o = zno + h₂↑. this chemistry video tutorial explains how to predict the products of the. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2. Zinc And Hydrochloric Acid Equation Balanced.
From slideplayer.com
Ch. 8 Chemical Equations and Reactions ppt download Zinc And Hydrochloric Acid Equation Balanced zn + 2h₂o = zno + h₂↑. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: this chemistry video tutorial explains how to predict the products of the. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. an explanation. Zinc And Hydrochloric Acid Equation Balanced.
From www.bartleby.com
Answered 3. Zinc and hydrochloric acid react… bartleby Zinc And Hydrochloric Acid Equation Balanced Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. zn + 2h₂o = zno + h₂↑. enter an equation of an ionic chemical equation and press the balance button. zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc sulfate [wikimedia] zinc is a. Zinc And Hydrochloric Acid Equation Balanced.
From www.chegg.com
Solved Choose balanced molecular, ionic, and net ionic Zinc And Hydrochloric Acid Equation Balanced zn + 2h₂o = zno + h₂↑. this chemistry video tutorial explains how to predict the products of the. Look here for some awesome experiments with zinc. an explanation of how to balance and write the the equation for zinc. this experiment includes the reaction of zinc with hydrochloric acid. zn + hcl = zncl2. Zinc And Hydrochloric Acid Equation Balanced.
From www.numerade.com
SOLVED Zinc reacts with hydrochloric acid according to the reaction Zinc And Hydrochloric Acid Equation Balanced zn + 2h₂o = zno + h₂↑. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. H₂so₄ + zn = znso₄ + h₂↑. this experiment includes the reaction of zinc with hydrochloric acid. this chemistry video tutorial explains how to predict the products of the. . Zinc And Hydrochloric Acid Equation Balanced.
From www.chemicals.co.uk
Practical GCSE Chemistry Making Salts The Science Blog Zinc And Hydrochloric Acid Equation Balanced The balanced equation will be calculated along. zn + 2h₂o = zno + h₂↑. The experiment compares reactivity of three. enter an equation of an ionic chemical equation and press the balance button. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: Zinc sulfate [wikimedia] zinc is a heavy metal compared. Zinc And Hydrochloric Acid Equation Balanced.
From www.youtube.com
How to balance zinc and hydrochloric acid? YouTube Zinc And Hydrochloric Acid Equation Balanced The balanced equation will be calculated along. in this video, we'll be exploring the reaction between zinc metal and. Look here for some awesome experiments with zinc. zinc metal reacts with hydrochloric acid according to the balanced equation: this chemistry video tutorial explains how to predict the products of the. If a piece of zinc is immersed. Zinc And Hydrochloric Acid Equation Balanced.
From www.chegg.com
Solved 1. Zinc metal reacts with hydrochloric acid according Zinc And Hydrochloric Acid Equation Balanced Zinc sulfate [wikimedia] zinc is a heavy metal compared with lithium, for instance, so the bubbles don’t rise to the surface. this experiment includes the reaction of zinc with hydrochloric acid. enter an equation of an ionic chemical equation and press the balance button. Look here for some awesome experiments with zinc. The experiment compares reactivity of three.. Zinc And Hydrochloric Acid Equation Balanced.
From www.numerade.com
SOLVEDZinc metal reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid Equation Balanced this chemistry video tutorial explains how to predict the products of the. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: in this video, we'll be exploring the reaction between zinc metal and. Look here for some awesome experiments with zinc. zinc metal reacts with hydrochloric acid according to the. Zinc And Hydrochloric Acid Equation Balanced.
From www.toppr.com
Zinc and hydrochloric acid react according to equation Zn(s) + 2HCl(aq Zinc And Hydrochloric Acid Equation Balanced Look here for some awesome experiments with zinc. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. in this video, we'll be exploring the reaction between zinc metal and. this experiment includes the reaction of zinc with hydrochloric acid. The experiment compares reactivity of three. zinc. Zinc And Hydrochloric Acid Equation Balanced.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Zinc And Hydrochloric Acid Equation Balanced zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. The experiment compares reactivity of three. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: this chemistry video tutorial explains how to predict the products of the. . Zinc And Hydrochloric Acid Equation Balanced.
From www.numerade.com
In this problem, zinc reacts with hydrochloric acid to make zinc Zinc And Hydrochloric Acid Equation Balanced zn + 2h₂o = zno + h₂↑. The balanced equation will be calculated along. zinc metal reacts with hydrochloric acid according to the balanced equation: this chemistry video tutorial explains how to predict the products of the. H₂so₄ + zn = znso₄ + h₂↑. an explanation of how to balance and write the the equation for. Zinc And Hydrochloric Acid Equation Balanced.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Zinc And Hydrochloric Acid Equation Balanced zinc metal reacts with hydrochloric acid according to the balanced equation: zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. H₂so₄ + zn = znso₄ + h₂↑. The experiment compares reactivity of three. enter an equation of an ionic chemical equation and press. Zinc And Hydrochloric Acid Equation Balanced.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid Equation Balanced Look here for some awesome experiments with zinc. this experiment includes the reaction of zinc with hydrochloric acid. zinc metal reacts with hydrochloric acid according to the balanced equation: zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. Zinc sulfate [wikimedia] zinc is. Zinc And Hydrochloric Acid Equation Balanced.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Zinc And Hydrochloric Acid Equation Balanced zn + 2h₂o = zno + h₂↑. this chemistry video tutorial explains how to predict the products of the. Look here for some awesome experiments with zinc. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: H₂so₄ + zn = znso₄ + h₂↑. Zinc sulfate [wikimedia] zinc is a heavy metal. Zinc And Hydrochloric Acid Equation Balanced.
From www.chegg.com
Solved Write the balanced chemical equation for the Zinc And Hydrochloric Acid Equation Balanced zn + 2h₂o = zno + h₂↑. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. Look here for some awesome experiments with zinc. enter an equation of an ionic chemical equation and press the balance button. zn + hcl = zncl2 + h2 is a. Zinc And Hydrochloric Acid Equation Balanced.
From www.numerade.com
SOLVED Consider the reaction of zinc metal with hydrochloric acid, HCl Zinc And Hydrochloric Acid Equation Balanced zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc sulfate [wikimedia] zinc is a heavy metal compared with lithium, for instance, so the bubbles don’t rise to the surface. The balanced equation will be calculated along. Look here for some awesome experiments with zinc. an explanation of how to balance and write the the equation. Zinc And Hydrochloric Acid Equation Balanced.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube Zinc And Hydrochloric Acid Equation Balanced H₂so₄ + zn = znso₄ + h₂↑. Look here for some awesome experiments with zinc. The experiment compares reactivity of three. zn + 2h₂o = zno + h₂↑. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. enter an equation of an ionic chemical equation and press. Zinc And Hydrochloric Acid Equation Balanced.
From www.transtutors.com
(Solved) Zinc Metal Reacts With Hydrochloric Acid According To The Zinc And Hydrochloric Acid Equation Balanced this experiment includes the reaction of zinc with hydrochloric acid. Look here for some awesome experiments with zinc. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. zinc metal reacts with hydrochloric acid according to the balanced equation: this chemistry video tutorial. Zinc And Hydrochloric Acid Equation Balanced.
From dxorwmpbw.blob.core.windows.net
Word Equation Between Zinc And Hydrochloric Acid at Noah Brooks blog Zinc And Hydrochloric Acid Equation Balanced Zinc sulfate [wikimedia] zinc is a heavy metal compared with lithium, for instance, so the bubbles don’t rise to the surface. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. enter an equation of an ionic chemical equation and press the balance button. Look here for some awesome. Zinc And Hydrochloric Acid Equation Balanced.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid Equation Balanced The balanced equation will be calculated along. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: The experiment compares reactivity of three. this chemistry video tutorial explains how to predict the products of the. H₂so₄ + zn = znso₄ + h₂↑. in this video, we'll be exploring the reaction between zinc. Zinc And Hydrochloric Acid Equation Balanced.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc And Hydrochloric Acid Equation Balanced Zinc sulfate [wikimedia] zinc is a heavy metal compared with lithium, for instance, so the bubbles don’t rise to the surface. this experiment includes the reaction of zinc with hydrochloric acid. in this video, we'll be exploring the reaction between zinc metal and. enter an equation of an ionic chemical equation and press the balance button. The. Zinc And Hydrochloric Acid Equation Balanced.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid Equation Balanced If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. The experiment compares reactivity of three. zn + 2h₂o = zno + h₂↑. Look here for some awesome experiments with zinc. zinc. Zinc And Hydrochloric Acid Equation Balanced.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid Equation Balanced enter an equation of an ionic chemical equation and press the balance button. zinc metal reacts with hydrochloric acid according to the balanced equation: in this video, we'll be exploring the reaction between zinc metal and. zn + 2h₂o = zno + h₂↑. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103. Zinc And Hydrochloric Acid Equation Balanced.
From www.slideshare.net
Acids And Bases Zinc And Hydrochloric Acid Equation Balanced Look here for some awesome experiments with zinc. The balanced equation will be calculated along. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. If a piece of zinc is immersed in diluted sulfuric acid, bubbles of hydrogen are released: this experiment includes the. Zinc And Hydrochloric Acid Equation Balanced.
From www.numerade.com
SOLVED A 15 g block of zinc is dissolved in 1 M hydrochloric acid (a Zinc And Hydrochloric Acid Equation Balanced Zinc sulfate [wikimedia] zinc is a heavy metal compared with lithium, for instance, so the bubbles don’t rise to the surface. in this video, we'll be exploring the reaction between zinc metal and. zinc metal reacts with hydrochloric acid according to the balanced equation: enter an equation of an ionic chemical equation and press the balance button.. Zinc And Hydrochloric Acid Equation Balanced.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc And Hydrochloric Acid Equation Balanced zn + 2h₂o = zno + h₂↑. this chemistry video tutorial explains how to predict the products of the. zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc sulfate [wikimedia] zinc is a heavy metal compared with lithium, for instance, so the bubbles don’t rise to the surface. enter an equation of an. Zinc And Hydrochloric Acid Equation Balanced.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc And Hydrochloric Acid Equation Balanced an explanation of how to balance and write the the equation for zinc. this chemistry video tutorial explains how to predict the products of the. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. The balanced equation will be calculated along. H₂so₄ +. Zinc And Hydrochloric Acid Equation Balanced.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + HCl = ZnCl2 + H2 YouTube Zinc And Hydrochloric Acid Equation Balanced this chemistry video tutorial explains how to predict the products of the. zinc metal reacts with hydrochloric acid according to the balanced equation: enter an equation of an ionic chemical equation and press the balance button. Look here for some awesome experiments with zinc. H₂so₄ + zn = znso₄ + h₂↑. zn + 2h₂o = zno. Zinc And Hydrochloric Acid Equation Balanced.
From slideplayer.com
Chemical Reactions & Equations ppt download Zinc And Hydrochloric Acid Equation Balanced Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. an explanation of how to balance and write the the equation for zinc. in this video, we'll be exploring the reaction between zinc metal and. this chemistry video tutorial explains how to predict the products of the.. Zinc And Hydrochloric Acid Equation Balanced.
From www.numerade.com
SOLVED The equation below shows the reaction of zinc with hydrochloric Zinc And Hydrochloric Acid Equation Balanced this chemistry video tutorial explains how to predict the products of the. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles. zinc metal. Zinc And Hydrochloric Acid Equation Balanced.