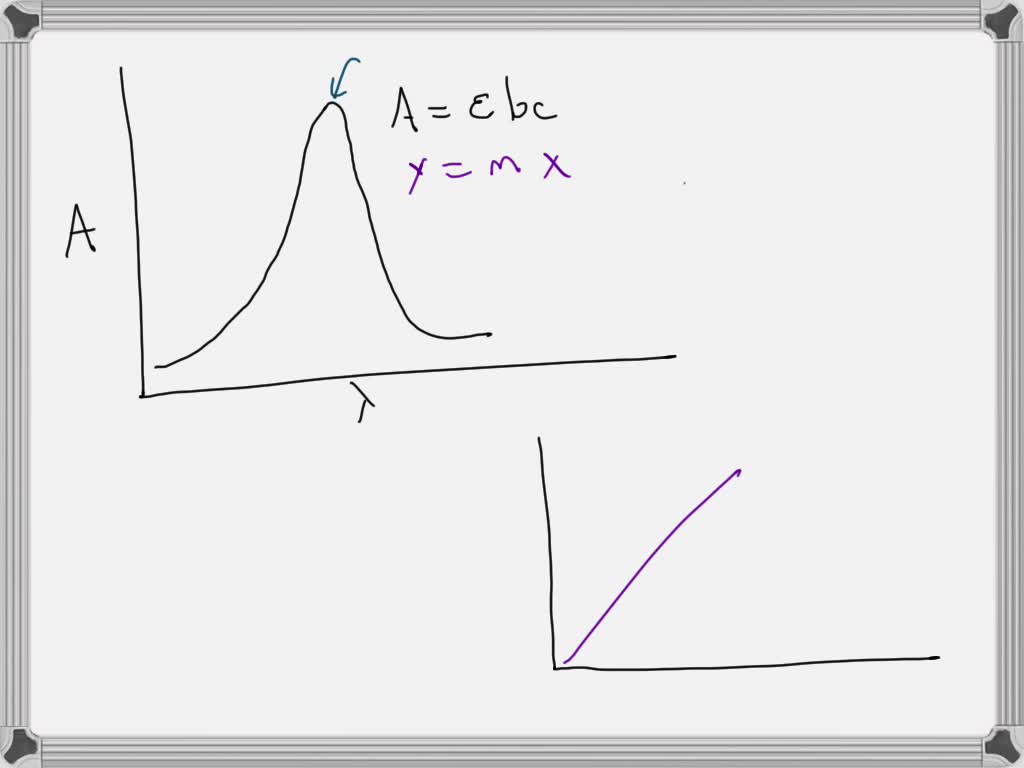
Beer's Law Y Intercept . Beer's law is expressed by a linear function, which relates. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. There are two contributions to this fundamental limitation to. In other words, a solution. Beer’s law suggests that a plot of absorbance vs. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Beer’s law is a limiting law that is valid only for low concentrations of analyte.
from www.numerade.com
Beer’s law suggests that a plot of absorbance vs. Beer’s law is a limiting law that is valid only for low concentrations of analyte. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. There are two contributions to this fundamental limitation to. Beer's law is expressed by a linear function, which relates. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions.
1. The equation for your line has a nonzero yintercept. Beer's law
Beer's Law Y Intercept Beer's law is expressed by a linear function, which relates. Beer's law is expressed by a linear function, which relates. Beer’s law is a limiting law that is valid only for low concentrations of analyte. In other words, a solution. Beer’s law suggests that a plot of absorbance vs. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. There are two contributions to this fundamental limitation to. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Y Intercept There are two contributions to this fundamental limitation to. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Beer’s law is a. Beer's Law Y Intercept.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Y Intercept Beer’s law suggests that a plot of absorbance vs. There are two contributions to this fundamental limitation to. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law is expressed by a linear function, which relates. Calculate the gradient (m) for the equation. Beer's Law Y Intercept.
From www.coursehero.com
[Solved] When you turn beers law into y=mx+b, what components of beers Beer's Law Y Intercept There are two contributions to this fundamental limitation to. Beer’s law suggests that a plot of absorbance vs. Beer’s law is a limiting law that is valid only for low concentrations of analyte. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law. Beer's Law Y Intercept.
From lelandchemclub.weebly.com
Solutions Leland Chemistry Club Beer's Law Y Intercept In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law suggests that a plot of absorbance vs. In other words, a solution. Beer's law is expressed by a linear function, which relates. There are two contributions to this fundamental limitation to. Calculate the. Beer's Law Y Intercept.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Y Intercept Beer’s law suggests that a plot of absorbance vs. There are two contributions to this fundamental limitation to. Beer’s law is a limiting law that is valid only for low concentrations of analyte. In other words, a solution. Beer's law is expressed by a linear function, which relates. In spectroscopy, beer’s law states that the absorption of light by a. Beer's Law Y Intercept.
From www.chegg.com
Solved Beer’s law is a linear relationship between Beer's Law Y Intercept Beer's law is expressed by a linear function, which relates. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Beer’s law suggests that a plot of absorbance vs. There are two contributions to this fundamental limitation to. In spectroscopy, beer’s law states that the absorption of light. Beer's Law Y Intercept.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Y Intercept There are two contributions to this fundamental limitation to. Beer’s law suggests that a plot of absorbance vs. Beer's law is expressed by a linear function, which relates. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Beer’s law. Beer's Law Y Intercept.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer's Law Y Intercept Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Beer’s law is a limiting law that is valid only for low concentrations of analyte. There are two contributions to this fundamental limitation to. Beer's law is expressed by a linear function, which relates. Beer’s law suggests that. Beer's Law Y Intercept.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law Y Intercept In other words, a solution. Beer's law is expressed by a linear function, which relates. Beer’s law is a limiting law that is valid only for low concentrations of analyte. There are two contributions to this fundamental limitation to. Beer’s law suggests that a plot of absorbance vs. In spectroscopy, beer’s law states that the absorption of light by a. Beer's Law Y Intercept.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Y Intercept There are two contributions to this fundamental limitation to. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Beer’s law suggests that a plot of absorbance vs. Beer's law is expressed by a linear function, which relates. Calculate the. Beer's Law Y Intercept.
From studylib.net
Beer`s Law Beer's Law Y Intercept There are two contributions to this fundamental limitation to. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law suggests that a plot of absorbance vs. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data. Beer's Law Y Intercept.
From stuff.iorodeo.com
Lab 2 Beer’s Law and Molar Extinction Coefficients — Colorimeter User Beer's Law Y Intercept Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Beer’s law is a limiting law that is valid only for low concentrations of analyte. There are two contributions to this fundamental limitation to. Beer's law is expressed by a linear function, which relates. In other words, a. Beer's Law Y Intercept.
From www.slideserve.com
PPT Spectroscopy PowerPoint Presentation, free download ID6249302 Beer's Law Y Intercept Beer’s law suggests that a plot of absorbance vs. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Calculate the gradient (m) for the. Beer's Law Y Intercept.
From www.numerade.com
SOLVED 3A. What is the mathematical expression of Beer's law? Explain Beer's Law Y Intercept There are two contributions to this fundamental limitation to. Beer's law is expressed by a linear function, which relates. In other words, a solution. Beer’s law is a limiting law that is valid only for low concentrations of analyte. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its. Beer's Law Y Intercept.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Y Intercept Beer’s law suggests that a plot of absorbance vs. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law is expressed by a linear function, which relates. In other words, a solution. Calculate the gradient (m) for the equation of the line of. Beer's Law Y Intercept.
From facts.net
13 Surprising Facts About BeerLambert Law Beer's Law Y Intercept Beer's law is expressed by a linear function, which relates. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law suggests that a plot of absorbance vs. In other words, a solution. Beer’s law is a limiting law that is valid only for. Beer's Law Y Intercept.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer's Law Y Intercept In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law is expressed by a linear function, which relates. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest. Beer's Law Y Intercept.
From www.slideserve.com
PPT Introduction to Spectroscopic Methods of Analysis (part 2 Beer's Law Y Intercept There are two contributions to this fundamental limitation to. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Beer's law is expressed by a linear function, which relates. In other words, a solution. Beer’s law suggests that a plot of absorbance vs. In spectroscopy, beer’s law states that the absorption of light by a. Beer's Law Y Intercept.
From thienvienchannguyen.net
Beer Lambert's Law, Absorbance \u0026 Transmittance Spectrophotometry Beer's Law Y Intercept Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Beer’s law suggests that a plot of absorbance vs. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Beer's Law Y Intercept.
From chem.libretexts.org
8.2 Beer's Law Chemistry LibreTexts Beer's Law Y Intercept Beer’s law suggests that a plot of absorbance vs. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law is expressed by a linear function, which relates. Calculate the gradient (m) for the equation of the line of. Beer's Law Y Intercept.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer's Law Y Intercept In other words, a solution. Beer’s law is a limiting law that is valid only for low concentrations of analyte. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Calculate the gradient (m) for the equation of the line of the calibration curve, using. Beer's Law Y Intercept.
From lelandchemclub.weebly.com
Leland Chemistry Club Leland Chemistry Club News Beer's Law Y Intercept There are two contributions to this fundamental limitation to. In other words, a solution. Beer’s law suggests that a plot of absorbance vs. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law is a limiting law that is valid only for low. Beer's Law Y Intercept.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer's Law Y Intercept Beer’s law suggests that a plot of absorbance vs. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Beer's law is expressed by a linear function, which relates. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. In spectroscopy, beer’s law. Beer's Law Y Intercept.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Beer's Law Y Intercept Beer’s law is a limiting law that is valid only for low concentrations of analyte. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. There are two contributions to this fundamental limitation to. Beer’s law suggests that a plot. Beer's Law Y Intercept.
From www.slideserve.com
PPT Beers Law for a Single Component Sample PowerPoint Presentation Beer's Law Y Intercept There are two contributions to this fundamental limitation to. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law is expressed by a linear function, which relates. Calculate the gradient (m) for the equation of the line of the calibration curve, using the. Beer's Law Y Intercept.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Y Intercept Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Beer's law is expressed by a linear function, which relates. In other words, a solution. There are two contributions to this fundamental limitation. Beer's Law Y Intercept.
From www.numerade.com
1. The equation for your line has a nonzero yintercept. Beer's law Beer's Law Y Intercept Beer's law is expressed by a linear function, which relates. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Beer’s law is a limiting law that is valid only for low concentrations of analyte. There are two contributions to. Beer's Law Y Intercept.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Beer's Law Y Intercept In other words, a solution. Beer’s law is a limiting law that is valid only for low concentrations of analyte. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law suggests that a plot of absorbance vs. Beer's law is expressed by a. Beer's Law Y Intercept.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Y Intercept Beer’s law suggests that a plot of absorbance vs. Beer’s law is a limiting law that is valid only for low concentrations of analyte. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. In other words, a solution. There are two contributions to this fundamental limitation to.. Beer's Law Y Intercept.
From www.slideshare.net
beer lamberts law, colorimetery, nephlometry and turbidimetry Beer's Law Y Intercept In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law is expressed by a linear function, which relates. In other words, a solution. Beer’s law suggests that a plot of absorbance vs. Beer’s law is a limiting law that is valid only for. Beer's Law Y Intercept.
From www.studocu.com
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the Beer's Law Y Intercept In other words, a solution. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Beer's law is expressed by a linear function, which relates. Beer’s law suggests that a plot of absorbance vs. Beer’s law is a limiting law that is valid only for low concentrations of. Beer's Law Y Intercept.
From www.chegg.com
Solved Describe what each term in the Beer's Law equation Beer's Law Y Intercept Beer’s law suggests that a plot of absorbance vs. Beer's law is expressed by a linear function, which relates. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. There are two contributions to this fundamental limitation to. In spectroscopy, beer’s law states that the absorption of light. Beer's Law Y Intercept.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Y Intercept There are two contributions to this fundamental limitation to. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Beer’s law suggests that. Beer's Law Y Intercept.
From www.youtube.com
Beer's Law Lab Procedure YouTube Beer's Law Y Intercept In other words, a solution. There are two contributions to this fundamental limitation to. Beer's law is expressed by a linear function, which relates. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Calculate the gradient (m) for the equation of the line of. Beer's Law Y Intercept.
From www.slideserve.com
PPT Spectrophotometric Determination of Iron Using 1,10 Beer's Law Y Intercept Beer's law is expressed by a linear function, which relates. There are two contributions to this fundamental limitation to. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Calculate the gradient (m) for the equation of the line of. Beer's Law Y Intercept.