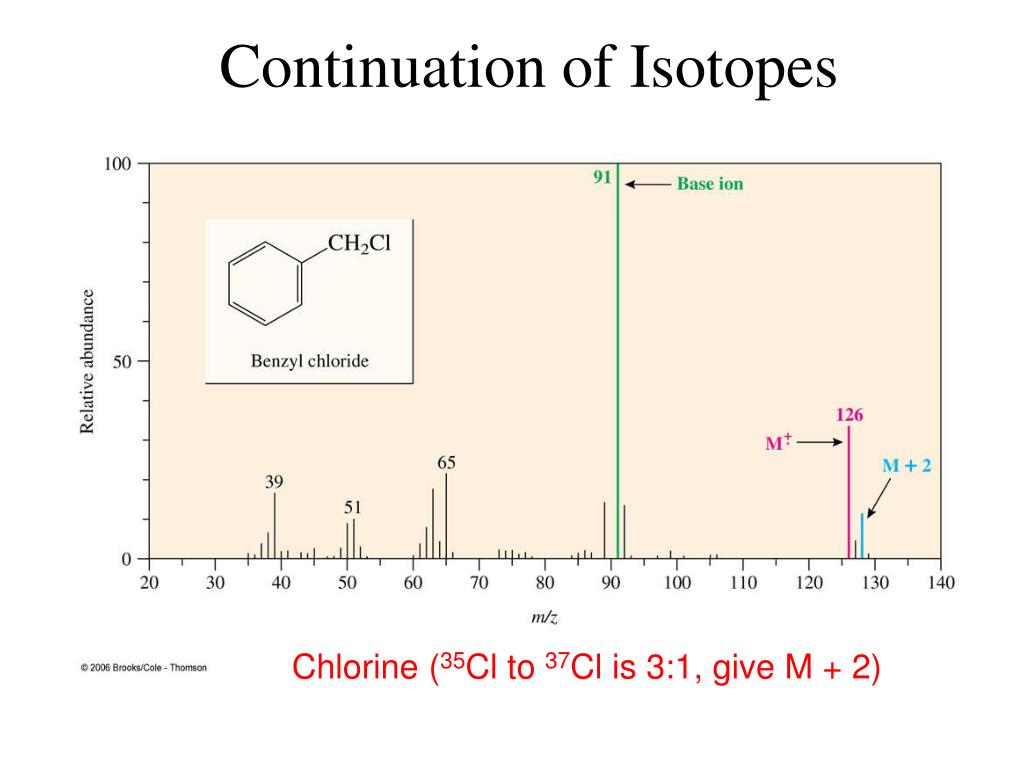
Chlorine Isotope Pattern Mass Spec . Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. There are two stable isotopes, 35 cl. All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. Find out the number, abundance and relative atomic mass of the isotopes of each. Please see the following for information about the library and its accompanying search.
from www.slideserve.com
Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. Find out the number, abundance and relative atomic mass of the isotopes of each. There are two stable isotopes, 35 cl. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have. All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Please see the following for information about the library and its accompanying search. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom.
PPT Mass Spectrometry (Mass Spec.) PowerPoint Presentation, free
Chlorine Isotope Pattern Mass Spec There are two stable isotopes, 35 cl. Find out the number, abundance and relative atomic mass of the isotopes of each. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. Please see the following for information about the library and its accompanying search. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: There are two stable isotopes, 35 cl. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have. All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library.
From www.docbrown.info
C2H4BrCl BrCH2CH2Cl mass spectrum of 1bromo2chloroethane Chlorine Isotope Pattern Mass Spec Please see the following for information about the library and its accompanying search. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50%. Chlorine Isotope Pattern Mass Spec.
From www.alamy.com
Isotopes of chlorine. Illustration showing the two principal stable Chlorine Isotope Pattern Mass Spec Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. Please see the following for information about the library and its accompanying search. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have.. Chlorine Isotope Pattern Mass Spec.
From www.sliderbase.com
Mass Spectrometry Presentation Chemistry Chlorine Isotope Pattern Mass Spec Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine. Chlorine Isotope Pattern Mass Spec.
From www.showme.com
Relative abundancies of Chlorine from a mass spectrometer Science Chlorine Isotope Pattern Mass Spec Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: Learn how. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine Isotope Pattern Mass Spec Please see the following for information about the library and its accompanying search. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. There are two stable isotopes, 35 cl. Find out the number, abundance and relative atomic mass of the isotopes of each.. Chlorine Isotope Pattern Mass Spec.
From chempedia.info
Chlorine isotope pattern Big Chemical Encyclopedia Chlorine Isotope Pattern Mass Spec There are two stable isotopes, 35 cl. All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. Find out the number, abundance and relative atomic mass of the isotopes of each. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a. Chlorine Isotope Pattern Mass Spec.
From www.chegg.com
Solved In mass spectrometry, there are patterns in the Chlorine Isotope Pattern Mass Spec Please see the following for information about the library and its accompanying search. There are two stable isotopes, 35 cl. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: Learn how to interpret the mass spectra of monatomic and diatomic elements, such as. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine Isotope Pattern Mass Spec All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. Please see the following for information about the library and its accompanying search. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. Learn how to interpret. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine Isotope Pattern Mass Spec The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. Please see the following for information about the library and its accompanying search. Find out the number, abundance and relative atomic mass of the isotopes of each. Learn how to interpret the mass spectra. Chlorine Isotope Pattern Mass Spec.
From www.researchgate.net
Comparison of mass values and isotopic patterns 1) experimental m/z Chlorine Isotope Pattern Mass Spec Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have. Find out the number, abundance and relative atomic mass of the isotopes of each. The natural abundance of these two isotopes is observed in the mass spectrum as. Chlorine Isotope Pattern Mass Spec.
From www.youtube.com
Chloro pattern in Mass Spectrometry YouTube Chlorine Isotope Pattern Mass Spec The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. There are two stable isotopes, 35 cl. Find out the number, abundance and relative atomic mass of the isotopes of each. Because there are two abundant isotopes of both chlorine (about 75% 35 cl. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT Relative atomic mass PowerPoint Presentation ID5933036 Chlorine Isotope Pattern Mass Spec The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have. Find out. Chlorine Isotope Pattern Mass Spec.
From www.youtube.com
13.04 Isotopic Abundance in Mass Spectrometry YouTube Chlorine Isotope Pattern Mass Spec Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: There are. Chlorine Isotope Pattern Mass Spec.
From www.docbrown.info
C4H9Cl (CH3)2CH2Cl mass spectrum of 1chloro2methylpropane Chlorine Isotope Pattern Mass Spec Find out the number, abundance and relative atomic mass of the isotopes of each. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52. Chlorine Isotope Pattern Mass Spec.
From www.researchgate.net
Examples of isotope patterns for two reference HAAs (DCAA and BCAA Chlorine Isotope Pattern Mass Spec Please see the following for information about the library and its accompanying search. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. The mass spectrum of. Chlorine Isotope Pattern Mass Spec.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Chlorine Isotope Pattern Mass Spec The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: There are two stable isotopes, 35 cl. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. Please. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT Mass Spectrometry (Mass Spec.) PowerPoint Presentation, free Chlorine Isotope Pattern Mass Spec The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. There are two stable isotopes, 35 cl. All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. Please see the following for information about the library and. Chlorine Isotope Pattern Mass Spec.
From www.docbrown.info
mass spectrum of 1chlorobutane fragmentation pattern of ions for Chlorine Isotope Pattern Mass Spec Find out the number, abundance and relative atomic mass of the isotopes of each. Please see the following for information about the library and its accompanying search. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. Because there are two abundant isotopes of. Chlorine Isotope Pattern Mass Spec.
From chem.libretexts.org
6.4 Isotope Abundance Chemistry LibreTexts Chlorine Isotope Pattern Mass Spec 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Find out the number, abundance and relative atomic mass of the isotopes of each. All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. There are two stable isotopes, 35. Chlorine Isotope Pattern Mass Spec.
From chempedia.info
Chlorine isotope pattern Big Chemical Encyclopedia Chlorine Isotope Pattern Mass Spec Find out the number, abundance and relative atomic mass of the isotopes of each. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. There are two stable isotopes, 35. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine Isotope Pattern Mass Spec 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are two stable isotopes, 35 cl. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: The mass spectrum of. Chlorine Isotope Pattern Mass Spec.
From www.researchgate.net
Stable isotopes of chlorine Download Scientific Diagram Chlorine Isotope Pattern Mass Spec 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. Find out the number, abundance and relative atomic mass of the. Chlorine Isotope Pattern Mass Spec.
From www.researchgate.net
Mass spectrometry experimental data (top) and isotopic pattern Chlorine Isotope Pattern Mass Spec The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: 53 rows chlorine (17 cl) has 25 isotopes,. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation ID2894903 Chlorine Isotope Pattern Mass Spec There are two stable isotopes, 35 cl. Please see the following for information about the library and its accompanying search. Find out the number, abundance and relative atomic mass of the isotopes of each. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom.. Chlorine Isotope Pattern Mass Spec.
From blog.sepscience.com
The Role of Isotope Peak Intensities Obtained Using Mass Spectrometry Chlorine Isotope Pattern Mass Spec There are two stable isotopes, 35 cl. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. All mass spectra in this site (plus many more) are available from. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation ID2894903 Chlorine Isotope Pattern Mass Spec Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. Please see the following for information about the library and its accompanying search. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. There are two stable isotopes, 35. Chlorine Isotope Pattern Mass Spec.
From kpu.docbrown.info
C6H5Cl mass spectrum of chlorobenzene fragmentation pattern of m/z m/e Chlorine Isotope Pattern Mass Spec The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. Find out the number, abundance and relative atomic mass of the isotopes of each. All mass spectra in this. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine Isotope Pattern Mass Spec Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: The mass. Chlorine Isotope Pattern Mass Spec.
From www.researchgate.net
Deuterium labeling causes predictable shifts in the isotope pattern Chlorine Isotope Pattern Mass Spec All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Please see the following for information about the library and its accompanying search. The natural abundance of these two isotopes. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT Why Mass Spectrometry An Introduction to the IU MSF PowerPoint Chlorine Isotope Pattern Mass Spec Find out the number, abundance and relative atomic mass of the isotopes of each. There are two stable isotopes, 35 cl. Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity. Chlorine Isotope Pattern Mass Spec.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Isotope Pattern Mass Spec Find out the number, abundance and relative atomic mass of the isotopes of each. Please see the following for information about the library and its accompanying search. All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope. Chlorine Isotope Pattern Mass Spec.
From www.researchgate.net
Mass spectra of products formed in the reaction of Hg 0 with molecular Chlorine Isotope Pattern Mass Spec There are two stable isotopes, 35 cl. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br),. Chlorine Isotope Pattern Mass Spec.
From www.researchgate.net
(a) Theoretical isotope pattern of [Mo2O6(OH)] ; (b) Experimental Chlorine Isotope Pattern Mass Spec The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: There are two stable isotopes, 35 cl. Learn how to interpret the mass spectra of monatomic and diatomic elements, such as chlorine. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl. Chlorine Isotope Pattern Mass Spec.
From www.slideserve.com
PPT Mass Spectrometry (Mass Spec.) PowerPoint Presentation, free Chlorine Isotope Pattern Mass Spec Find out the number, abundance and relative atomic mass of the isotopes of each. There are two stable isotopes, 35 cl. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have. 53 rows chlorine (17 cl) has 25. Chlorine Isotope Pattern Mass Spec.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Chlorine Isotope Pattern Mass Spec 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The mass spectrum of ch 3 cl (figure 21) clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom. The natural abundance of these two isotopes is observed in the. Chlorine Isotope Pattern Mass Spec.