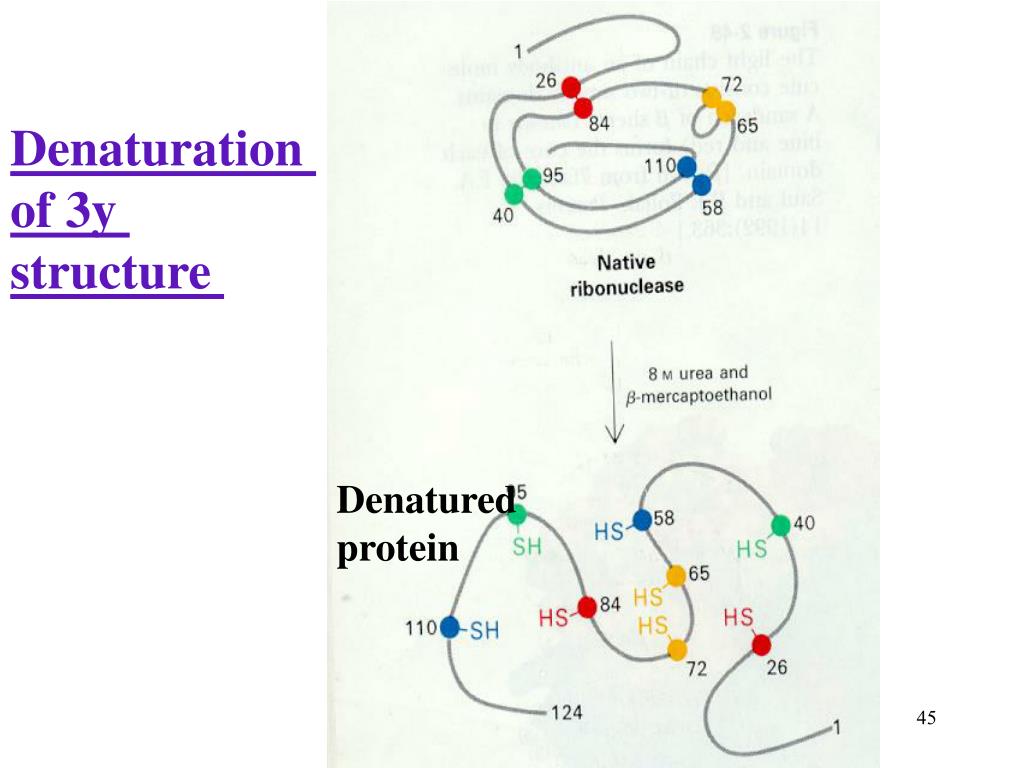
Protein Denaturation Enzymes . Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. Four interactions stabilize the tertiary structure of a protein: The primary structures of proteins are quite sturdy. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. Some proteins can refold after. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds.
from www.slideserve.com
The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. The primary structures of proteins are quite sturdy. Four interactions stabilize the tertiary structure of a protein: Some proteins can refold after. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing.
PPT PROTEINS & ENZYMES PowerPoint Presentation, free download ID
Protein Denaturation Enzymes The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Some proteins can refold after. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The primary structures of proteins are quite sturdy. The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. Four interactions stabilize the tertiary structure of a protein: (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d).
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID1395419 Protein Denaturation Enzymes Four interactions stabilize the tertiary structure of a protein: The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. Some proteins can refold after. (a) ionic bonding,. Protein Denaturation Enzymes.
From www.animalia-life.club
Denatured Enzyme Protein Denaturation Enzymes (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). Some proteins can refold after. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. The primary structures of proteins are quite sturdy. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The body. Protein Denaturation Enzymes.
From www.studocu.com
Protein Denaturation in Labster PROTEIN DENATURATION is the Protein Denaturation Enzymes The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. Protein enzymes involved in disulfide bond formation contain free cys which form mixed. Protein Denaturation Enzymes.
From byjus.com
During the denaturation of proteins, which of these structures will Protein Denaturation Enzymes Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). Some proteins can refold after. The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. The body strictly regulates ph and temperature to prevent proteins such as enzymes from. Protein Denaturation Enzymes.
From www.slideserve.com
PPT Denaturation of Enzymes PowerPoint Presentation, free download Protein Denaturation Enzymes Four interactions stabilize the tertiary structure of a protein: Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. The primary structures of. Protein Denaturation Enzymes.
From www.sciencelearn.org.nz
Denatured enzyme — Science Learning Hub Protein Denaturation Enzymes Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. The primary structures of proteins are quite sturdy. The red boxes represent stabilizing interactions, such. Protein Denaturation Enzymes.
From www.slideserve.com
PPT PROTEINS & ENZYMES PowerPoint Presentation, free download ID Protein Denaturation Enzymes Four interactions stabilize the tertiary structure of a protein: The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. Some proteins can refold after. The body strictly regulates ph and temperature to prevent proteins such as. Protein Denaturation Enzymes.
From www.slideshare.net
Protein structure and shape, Denaturation and Enzymes Protein Denaturation Enzymes (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The body. Protein Denaturation Enzymes.
From byjus.com
Denaturation Of Proteins By Urea Enzymes Of Denatured Ph Value Protein Denaturation Enzymes Some proteins can refold after. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). Four interactions stabilize the tertiary structure of a protein: The primary structures of proteins are quite sturdy. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The denaturation (unfolding) and renaturation (refolding) of a protein is. Protein Denaturation Enzymes.
From ppt-online.org
Protein denaturation презентация онлайн Protein Denaturation Enzymes The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). Some proteins can refold after. The body strictly regulates ph and temperature to prevent proteins such as enzymes from. Protein Denaturation Enzymes.
From montessorimuddle.org
food science Montessori Muddle Protein Denaturation Enzymes Some proteins can refold after. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. The primary structures of proteins are quite sturdy. Four interactions stabilize the. Protein Denaturation Enzymes.
From www.slideserve.com
PPT Denaturation of Enzymes PowerPoint Presentation, free download Protein Denaturation Enzymes The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Four interactions stabilize the tertiary structure of a protein: The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. Some proteins can refold after. (a) ionic bonding, (b) hydrogen. Protein Denaturation Enzymes.
From openoregon.pressbooks.pub
Protein Digestion and Absorption Nutrition Science and Everyday Protein Denaturation Enzymes Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The denaturation (unfolding) and renaturation (refolding) of a protein. Protein Denaturation Enzymes.
From openoregon.pressbooks.pub
Protein Digestion and Absorption Nutrition Science and Everyday Protein Denaturation Enzymes Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The red boxes represent stabilizing interactions, such as disulfide. Protein Denaturation Enzymes.
From slideplayer.com
Chemical Reactions, Energy, and Enzymes ppt download Protein Denaturation Enzymes The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. The primary structures of proteins are quite sturdy. Four interactions stabilize the tertiary structure of a protein: Some proteins can refold after. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide. Protein Denaturation Enzymes.
From www.differencebetween.com
Difference Between Protein Denaturation and Hydrolysis Compare the Protein Denaturation Enzymes Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Some proteins can refold after. The body strictly regulates ph and temperature to. Protein Denaturation Enzymes.
From www.slideserve.com
PPT 3.2 & 7.5 Proteins PowerPoint Presentation, free download ID Protein Denaturation Enzymes Some proteins can refold after. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. Four interactions stabilize the tertiary structure of a protein: Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides. Protein Denaturation Enzymes.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID2045802 Protein Denaturation Enzymes The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. Four interactions stabilize the tertiary structure of a protein: Some proteins can refold after. The primary structures of proteins are quite sturdy. Proteins are denatured by treatment with alkaline or. Protein Denaturation Enzymes.
From flatworldknowledge.lardbucket.org
Proteins Protein Denaturation Enzymes The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. Proteins are denatured by treatment with alkaline or acid, oxidizing or. Protein Denaturation Enzymes.
From sites.google.com
13chapter (biochemistry ) chemistry.edu.ssc.shahid mahmood Protein Denaturation Enzymes Some proteins can refold after. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The body strictly regulates ph and temperature to prevent proteins. Protein Denaturation Enzymes.
From www.slideshare.net
Protein structure and shape, Denaturation and Enzymes Protein Denaturation Enzymes Some proteins can refold after. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate. Protein Denaturation Enzymes.
From www.labster.com
5 Ways to Make Protein Denaturation A More Approachable Topic Protein Denaturation Enzymes The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target. Protein Denaturation Enzymes.
From www.youtube.com
Chapter 16, part 2 Protein denaturation and Enzymes YouTube Protein Denaturation Enzymes Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The primary structures of proteins are quite sturdy. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. (a). Protein Denaturation Enzymes.
From slideplayer.com
Proteins From Foods to Cells in the Body ppt download Protein Denaturation Enzymes Some proteins can refold after. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. The primary structures of proteins are quite sturdy. Protein enzymes involved in disulfide bond. Protein Denaturation Enzymes.
From www.youtube.com
Optimum rate and denatured enzymes YouTube Protein Denaturation Enzymes The primary structures of proteins are quite sturdy. Four interactions stabilize the tertiary structure of a protein: Some proteins can refold after. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Protein. Protein Denaturation Enzymes.
From www.lecturio.de
Posttranslationale Proteinmodifikationen Strukturen Lecturio Protein Denaturation Enzymes The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Some proteins can refold after. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. Protein. Protein Denaturation Enzymes.
From www.foodnetworksolution.com
Protein denaturation / การสูญเสียสภาพธรรมชาติของโปรตีน Food Wiki Protein Denaturation Enzymes Some proteins can refold after. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Four interactions stabilize the tertiary structure of a protein: The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. The primary structures. Protein Denaturation Enzymes.
From studylib.net
denature proteinsenzymes Protein Denaturation Enzymes Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. Some proteins can refold after. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The primary structures of proteins are quite sturdy. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d).. Protein Denaturation Enzymes.
From www.animalia-life.club
Denatured Enzyme Protein Denaturation Enzymes Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The primary structures of proteins are quite sturdy. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). The body strictly regulates ph. Protein Denaturation Enzymes.
From www.researchgate.net
Protein denaturation via enzyme interaction identified using Sypro Protein Denaturation Enzymes The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Four interactions. Protein Denaturation Enzymes.
From ar.inspiredpencil.com
Denatured Protein Protein Denaturation Enzymes Some proteins can refold after. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). Four interactions stabilize the tertiary structure of a protein: Protein enzymes involved. Protein Denaturation Enzymes.
From www.slideserve.com
PPT ENZYME CATALYSIS LAB PowerPoint Presentation, free download ID Protein Denaturation Enzymes Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain organic solvents. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. (a) ionic bonding, (b) hydrogen bonding, (c) disulfide linkages, and (d). The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. Four interactions stabilize the tertiary structure. Protein Denaturation Enzymes.
From en.ppt-online.org
Protein denaturation online presentation Protein Denaturation Enzymes Four interactions stabilize the tertiary structure of a protein: Some proteins can refold after. The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. The primary structures of proteins are quite sturdy. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding,. Protein Denaturation Enzymes.
From theory.labster.com
Definizione della denaturazione delle proteine Labster Theory Protein Denaturation Enzymes Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds. Proteins are denatured by treatment with alkaline or acid, oxidizing or reducing agents, and certain. Protein Denaturation Enzymes.
From chemistrytalk.org
Denaturation of Proteins What is it? ChemTalk Protein Denaturation Enzymes Protein enzymes involved in disulfide bond formation contain free cys which form mixed disulfides with their target substrate proteins. Four interactions stabilize the tertiary structure of a protein: The primary structures of proteins are quite sturdy. Some proteins can refold after. The body strictly regulates ph and temperature to prevent proteins such as enzymes from denaturing. The red boxes represent. Protein Denaturation Enzymes.