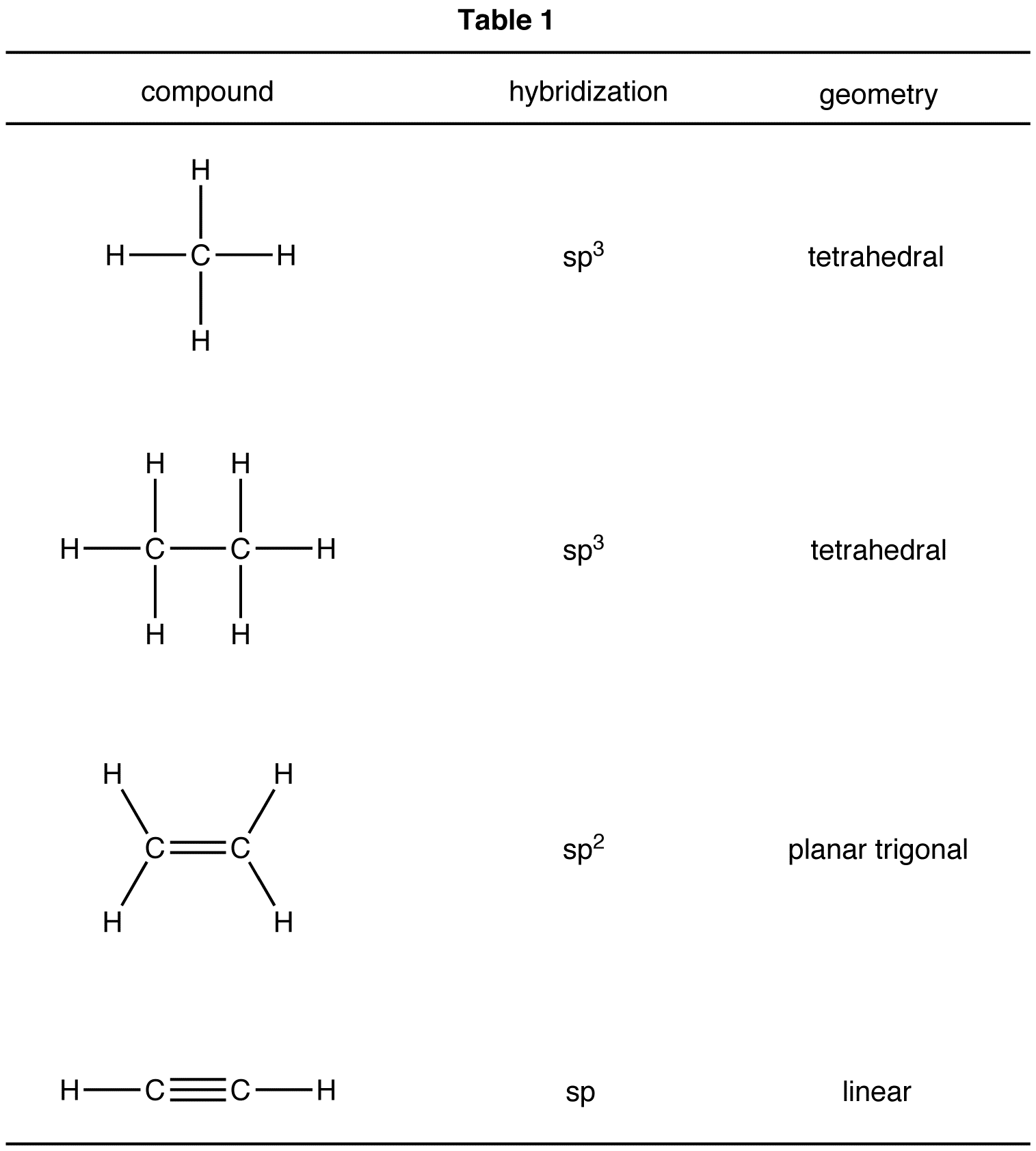
Steps Of Hybridization . usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). Today’s post is all about hybrid orbitals. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. ionic or electrovalent compounds. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. In it, the 2s orbital and one of the 2p orbitals. Sp hybridization can explain the linear structure in molecules.
from chem.libretexts.org
hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. Today’s post is all about hybrid orbitals. Sp hybridization can explain the linear structure in molecules. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. In it, the 2s orbital and one of the 2p orbitals. The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). ionic or electrovalent compounds.
Hybridization Chemistry LibreTexts
Steps Of Hybridization ionic or electrovalent compounds. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. In it, the 2s orbital and one of the 2p orbitals. Sp hybridization can explain the linear structure in molecules. Today’s post is all about hybrid orbitals. ionic or electrovalent compounds. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs).
From bio3400.nicerweb.net
molecular_hybridization.html 10_21molecular_hybridization.jpg Steps Of Hybridization hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. ionic or electrovalent compounds. The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. Today’s post is all about hybrid orbitals. In it, the 2s. Steps Of Hybridization.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps Steps Of Hybridization hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. Today’s post is all about hybrid orbitals. Sp hybridization can explain the linear structure in molecules. In it, the 2s orbital. Steps Of Hybridization.
From chemistrytalk.org
Hybridization and Hybrid Orbitals ChemTalk Steps Of Hybridization usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. Sp hybridization can explain the linear structure in molecules. In it, the 2s orbital and one of. Steps Of Hybridization.
From www.youtube.com
Steps of Artificial Hybridization YouTube Steps Of Hybridization hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. Sp hybridization can explain the linear structure in molecules. In it, the 2s orbital and one of the 2p orbitals. . Steps Of Hybridization.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Steps Of Hybridization The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. ionic or electrovalent compounds. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. hybridization is the process of combining pure atomic orbitals on. Steps Of Hybridization.
From www.researchgate.net
Basic DNA reactions. a A process of hybridization. Two DNA strands Steps Of Hybridization ionic or electrovalent compounds. Sp hybridization can explain the linear structure in molecules. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). In it, the 2s orbital and one of the 2p orbitals. The total number of electron groups just equals the total. Steps Of Hybridization.
From www.youtube.com
Chemistry 11Chapter 4 Chemical Bonding &Molecular Structure Steps Of Hybridization In it, the 2s orbital and one of the 2p orbitals. Sp hybridization can explain the linear structure in molecules. Today’s post is all about hybrid orbitals. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. The total number of electron groups just. Steps Of Hybridization.
From www.youtube.com
Somatic hybridization/Steps/process of fusion of protoplast of somatic Steps Of Hybridization ionic or electrovalent compounds. The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. In it, the 2s orbital and one of the 2p orbitals.. Steps Of Hybridization.
From www.researchgate.net
Main steps of the genomic in situ hybridization (GISH). (A) Direct and Steps Of Hybridization hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. Today’s post is all about hybrid orbitals. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same. Steps Of Hybridization.
From chemistnotes.com
Hybridization Definition, types and examples Chemistry Notes Steps Of Hybridization The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. Today’s post is all about hybrid orbitals. ionic or electrovalent compounds. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the. Steps Of Hybridization.
From www.slideserve.com
PPT DNA Probing PowerPoint Presentation, free download ID2110493 Steps Of Hybridization Sp hybridization can explain the linear structure in molecules. ionic or electrovalent compounds. In it, the 2s orbital and one of the 2p orbitals. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The total number of electron groups just equals the total. Steps Of Hybridization.
From www.geeksforgeeks.org
Hybridization Definition, Types, Rules, Examples Steps Of Hybridization In it, the 2s orbital and one of the 2p orbitals. Today’s post is all about hybrid orbitals. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy. Steps Of Hybridization.
From www.chemohollic.com
Hybridisation in Five Simple Steps All 'Bout Chemistry Steps Of Hybridization The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. usually the. Steps Of Hybridization.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Steps Of Hybridization Today’s post is all about hybrid orbitals. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). In it, the 2s orbital and one of the 2p orbitals. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy. Steps Of Hybridization.
From www.animalia-life.club
Sp3d Orbitals Steps Of Hybridization Today’s post is all about hybrid orbitals. ionic or electrovalent compounds. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. In it, the 2s orbital and one of the 2p orbitals. Sp hybridization can explain the linear structure in molecules. The total. Steps Of Hybridization.
From everchem.com.my
Hybrid Plants What are they and how are they hybridized? Everchem Steps Of Hybridization The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal. Steps Of Hybridization.
From www.researchgate.net
Basic steps of fluorescent in situ hybridization technique DNA Steps Of Hybridization The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. Sp hybridization can explain the linear structure in molecules. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). Today’s post is all about hybrid orbitals. . Steps Of Hybridization.
From mammothmemory.net
Detailed diagram and mnemonic of DNA hybridisation Steps Of Hybridization hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. In it, the 2s orbital and one of the 2p orbitals. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and. Steps Of Hybridization.
From www.cureus.com
Cureus Application of Fluorescence In Situ Hybridization (FISH Steps Of Hybridization ionic or electrovalent compounds. Today’s post is all about hybrid orbitals. In it, the 2s orbital and one of the 2p orbitals. The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form. Steps Of Hybridization.
From www.slideserve.com
PPT SOMATIC HYBRIDIZATION PowerPoint Presentation ID784032 Steps Of Hybridization hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy,. Steps Of Hybridization.
From www.youtube.com
Steps of HYBRIDIZATION TECHNIQUES YouTube Steps Of Hybridization ionic or electrovalent compounds. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. In it, the 2s orbital and one of the 2p orbitals. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to. Steps Of Hybridization.
From www.geeksforgeeks.org
Hybridization Definition, Types, Rules, Examples Steps Of Hybridization Sp hybridization can explain the linear structure in molecules. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. Today’s post is all about hybrid orbitals. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to. Steps Of Hybridization.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps Steps Of Hybridization hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. In it, the 2s orbital and one of the 2p orbitals. ionic or electrovalent compounds. Today’s post is all about hybrid orbitals. The total number of electron groups just equals the total number. Steps Of Hybridization.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Steps Of Hybridization Today’s post is all about hybrid orbitals. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number. Steps Of Hybridization.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry Steps Of Hybridization usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. Today’s post is all about hybrid orbitals. ionic. Steps Of Hybridization.
From www.researchgate.net
General steps in somatic hybridization Download Scientific Diagram Steps Of Hybridization hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. In it, the 2s orbital and one of the 2p orbitals. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and. Steps Of Hybridization.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps Steps Of Hybridization Today’s post is all about hybrid orbitals. Sp hybridization can explain the linear structure in molecules. In it, the 2s orbital and one of the 2p orbitals. usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). hybridization is defined as the phenomenon of. Steps Of Hybridization.
From www.ochempal.org
Hybridization OChemPal Steps Of Hybridization Today’s post is all about hybrid orbitals. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. Sp hybridization can explain the linear structure in molecules. In it, the 2s orbital and one of the 2p orbitals. ionic or electrovalent compounds. The total. Steps Of Hybridization.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Steps Of Hybridization usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy,. Steps Of Hybridization.
From www.researchgate.net
Flow chart showing the methodological steps of the hybridization Steps Of Hybridization hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. hybridization is. Steps Of Hybridization.
From www.researchgate.net
Steps to identify hybridization with genome exclusion. The dashed Steps Of Hybridization hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy,. Steps Of Hybridization.
From geneticeducation.co.in
What is DNA hybridization and How does it occur? Education Steps Of Hybridization In it, the 2s orbital and one of the 2p orbitals. The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing. Steps Of Hybridization.
From www.crackchemistry.in
Tricks to find the shape and hybridization of molecule Steps Of Hybridization Sp hybridization can explain the linear structure in molecules. Today’s post is all about hybrid orbitals. hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. hybridization is defined as. Steps Of Hybridization.
From plantbible.blogspot.com
Hybridization Steps, Types of hybridization Steps Of Hybridization Sp hybridization can explain the linear structure in molecules. Today’s post is all about hybrid orbitals. The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. In it, the 2s orbital and one of the 2p orbitals. ionic or electrovalent compounds. hybridization is the process of combining pure atomic orbitals. Steps Of Hybridization.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Steps Of Hybridization Sp hybridization can explain the linear structure in molecules. hybridization is defined as the phenomenon of mixing up (or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new. The total number of electron groups just equals the total number of orbitals involved in the certain hybridization. usually the hybridization on a certain. Steps Of Hybridization.