Arrhenius Equation Calculator Two Temperatures . It provides insights into how varying temperatures affect the speed of chemical reactions. This article will explore the arrhenius. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. The arrhenius equation can be used to compare the rate constants (k) of a. Arrhenius activation energy calculator for two temperatures. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). This activation energy calculator (also called the arrhenius equation calculator can help you calculate the minimum energy required for a chemical reaction to happen. The arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: What is the arrhenius equation for two temperatures?
from www.numerade.com
Arrhenius activation energy calculator for two temperatures. This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). This article will explore the arrhenius. The arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. It provides insights into how varying temperatures affect the speed of chemical reactions. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: What is the arrhenius equation for two temperatures? The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. The arrhenius equation can be used to compare the rate constants (k) of a.
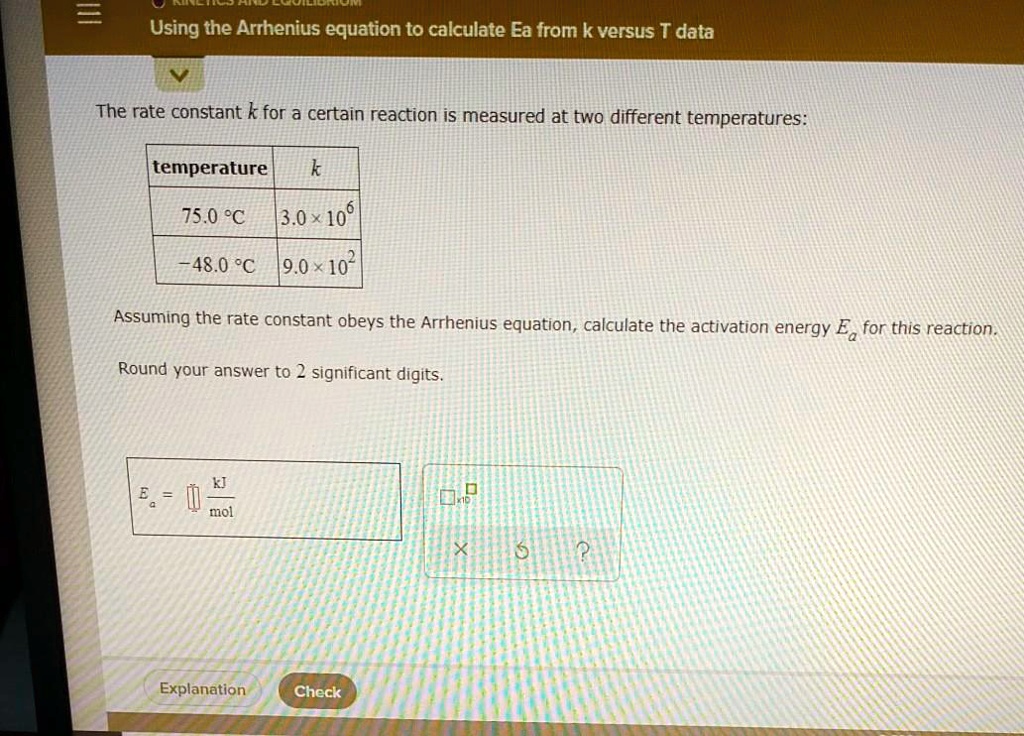
SOLVED Using the Arrhenius equation to calculate Ea from k versus T
Arrhenius Equation Calculator Two Temperatures This activation energy calculator (also called the arrhenius equation calculator can help you calculate the minimum energy required for a chemical reaction to happen. This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. This article will explore the arrhenius. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: Arrhenius activation energy calculator for two temperatures. The arrhenius equation can be used to compare the rate constants (k) of a. This activation energy calculator (also called the arrhenius equation calculator can help you calculate the minimum energy required for a chemical reaction to happen. The arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). It provides insights into how varying temperatures affect the speed of chemical reactions. What is the arrhenius equation for two temperatures?
From www.numerade.com
SOLVED The rate constant for certain reaction is measured at two Arrhenius Equation Calculator Two Temperatures What is the arrhenius equation for two temperatures? Arrhenius activation energy calculator for two temperatures. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). This article will explore the arrhenius. This activation energy calculator (also called the arrhenius equation calculator can help you calculate the minimum energy required for a chemical reaction. Arrhenius Equation Calculator Two Temperatures.
From www.youtube.com
Arrhenius Equation 2 data points to solve for k2 or Ea YouTube Arrhenius Equation Calculator Two Temperatures What is the arrhenius equation for two temperatures? The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. This article will explore the arrhenius. The arrhenius equation can be used to compare the rate constants (k) of a. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin,. Arrhenius Equation Calculator Two Temperatures.
From chemenggcalc.com
Arrhenius Activation Energy Calculator for two temperatures ChemEnggCalc Arrhenius Equation Calculator Two Temperatures The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. What is the arrhenius equation for two temperatures? The arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. The arrhenius equation can be used to compare the rate constants (k) of. Arrhenius Equation Calculator Two Temperatures.
From www.numerade.com
SOLVED The Arrhenius equation shows the relationship between the rate Arrhenius Equation Calculator Two Temperatures Arrhenius activation energy calculator for two temperatures. The arrhenius equation can be used to compare the rate constants (k) of a. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. This activation energy calculator (also called the arrhenius equation calculator can help you calculate the minimum energy required for a chemical reaction. Arrhenius Equation Calculator Two Temperatures.
From calculatorey.com
Arrhenius Equation Calculator Two Temperatures Calculatorey Arrhenius Equation Calculator Two Temperatures The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. The arrhenius equation can be used to compare the rate constants (k) of a. It provides insights into how varying temperatures affect the speed of chemical reactions. This activation energy calculator (also called the arrhenius equation calculator can help you calculate the minimum. Arrhenius Equation Calculator Two Temperatures.
From www.numerade.com
SOLVED Using the Arrhenius equation to calculate Ea from k versus T Arrhenius Equation Calculator Two Temperatures This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. It provides insights into how varying temperatures affect the speed of chemical reactions. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures:. Arrhenius Equation Calculator Two Temperatures.
From www.slideserve.com
PPT The Arrhenius Equation PowerPoint Presentation, free download Arrhenius Equation Calculator Two Temperatures Arrhenius activation energy calculator for two temperatures. This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. What is the arrhenius equation for two temperatures? The arrhenius equation can be used to compare the rate constants (k) of a. This activation energy calculator (also called the arrhenius equation. Arrhenius Equation Calculator Two Temperatures.
From www.numerade.com
SOLVED AND EQUILIBRIUM Using the Arrhenius equation to Arrhenius Equation Calculator Two Temperatures What is the arrhenius equation for two temperatures? This article will explore the arrhenius. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: Arrhenius activation energy calculator for two temperatures. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute. Arrhenius Equation Calculator Two Temperatures.
From www.numerade.com
SOLVED ' The rate constant k for a certain reaction is measured at two Arrhenius Equation Calculator Two Temperatures The arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. The arrhenius equation can. Arrhenius Equation Calculator Two Temperatures.
From www.youtube.com
16.3e Using the Arrhenius equation to calculate Ea from k versus T data Arrhenius Equation Calculator Two Temperatures What is the arrhenius equation for two temperatures? Arrhenius activation energy calculator for two temperatures. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. Below is an arrhenius equation calculator that allows you to input. Arrhenius Equation Calculator Two Temperatures.
From www.dreamstime.com
Arrhenius Equation Physical Chemistry Science Vector Infographic Stock Arrhenius Equation Calculator Two Temperatures Arrhenius activation energy calculator for two temperatures. This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. The arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. The arrhenius equation can be used to compare the. Arrhenius Equation Calculator Two Temperatures.
From www.numerade.com
SOLVEDKinGTCS AND EQUILIARIUM Using the Arrhenius equation to Arrhenius Equation Calculator Two Temperatures Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: The arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. This activation energy calculator (also called the arrhenius equation calculator can help you. Arrhenius Equation Calculator Two Temperatures.
From general.chemistrysteps.com
Arrhenius Equation Chemistry Steps Arrhenius Equation Calculator Two Temperatures What is the arrhenius equation for two temperatures? The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: The arrhenius equation is a formula that describes how the. Arrhenius Equation Calculator Two Temperatures.
From www.numerade.com
SOLVED ANDEQULIBRTUM Using the Arrhenius equation to Arrhenius Equation Calculator Two Temperatures This article will explore the arrhenius. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. What is the arrhenius equation for two temperatures? Arrhenius activation energy calculator for two temperatures.. Arrhenius Equation Calculator Two Temperatures.
From www.coursehero.com
[Solved] Alternate Form of the Arrhenius Equation At two temperatures Arrhenius Equation Calculator Two Temperatures This article will explore the arrhenius. This activation energy calculator (also called the arrhenius equation calculator can help you calculate the minimum energy required for a chemical reaction to happen. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. Arrhenius activation energy calculator for two temperatures. The arrhenius equation can be used. Arrhenius Equation Calculator Two Temperatures.
From www.numerade.com
SOLVED AND EQUILIBRIUM Using the Arrhenius equation to Arrhenius Equation Calculator Two Temperatures It provides insights into how varying temperatures affect the speed of chemical reactions. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. The arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. This article will explore the arrhenius. Users input. Arrhenius Equation Calculator Two Temperatures.
From www.slideserve.com
PPT Chapter 14 – Chemical PowerPoint Presentation, free Arrhenius Equation Calculator Two Temperatures Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: The arrhenius equation can be used to compare the rate constants (k) of a. This article will explore the arrhenius. This activation energy calculator (also called the arrhenius equation calculator can help you calculate the. Arrhenius Equation Calculator Two Temperatures.
From www.numerade.com
SOLVED Stephanie Using the Arrhenius equation t0 calculate Ea from The Arrhenius Equation Calculator Two Temperatures Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. The arrhenius equation is a formula that describes how the rate of a. Arrhenius Equation Calculator Two Temperatures.
From www.chegg.com
Solved TwoPoint Form of Arrhenius Equation In N2. Arrhenius Equation Calculator Two Temperatures The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). The arrhenius equation can be used to compare the rate constants (k) of a. What is the arrhenius equation for two temperatures? The arrhenius equation is. Arrhenius Equation Calculator Two Temperatures.
From www.youtube.com
Temperature and Arrhenius equation YouTube Arrhenius Equation Calculator Two Temperatures Arrhenius activation energy calculator for two temperatures. It provides insights into how varying temperatures affect the speed of chemical reactions. This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). This. Arrhenius Equation Calculator Two Temperatures.
From www.slideserve.com
PPT Arrhenius equation PowerPoint Presentation, free download ID Arrhenius Equation Calculator Two Temperatures The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). It provides insights into how varying temperatures affect the speed of chemical reactions. This activation energy calculator is helpful for users to determine the activation energy. Arrhenius Equation Calculator Two Temperatures.
From www.researchgate.net
Arrhenius Parameter (A) in the Two Stages at Different Temperatures Arrhenius Equation Calculator Two Temperatures It provides insights into how varying temperatures affect the speed of chemical reactions. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: What is the arrhenius equation for two temperatures? Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius,. Arrhenius Equation Calculator Two Temperatures.
From byjus.com
arrhenius equation Arrhenius Equation Calculator Two Temperatures It provides insights into how varying temperatures affect the speed of chemical reactions. This article will explore the arrhenius. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. Arrhenius activation energy calculator for two temperatures. This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius. Arrhenius Equation Calculator Two Temperatures.
From general.chemistrysteps.com
Arrhenius Equation Chemistry Steps Arrhenius Equation Calculator Two Temperatures The arrhenius equation can be used to compare the rate constants (k) of a. The arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. Below is an arrhenius equation calculator that. Arrhenius Equation Calculator Two Temperatures.
From www.savemyexams.com
The Arrhenius Equation OCR A Level Chemistry Revision Notes 2017 Arrhenius Equation Calculator Two Temperatures The arrhenius equation can be used to compare the rate constants (k) of a. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. This activation energy calculator (also called the arrhenius equation calculator can help you calculate the minimum energy required for a chemical reaction to happen. The arrhenius equation is a. Arrhenius Equation Calculator Two Temperatures.
From studylib.net
Arrhenius Equation Arrhenius Equation Calculator Two Temperatures The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. The arrhenius equation can be used to compare the rate constants (k) of a. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: It provides insights into. Arrhenius Equation Calculator Two Temperatures.
From www.chegg.com
Solved 6. The Arrhenius equation is used to calculate the Arrhenius Equation Calculator Two Temperatures This article will explore the arrhenius. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: The arrhenius equation can be used to compare the rate constants (k). Arrhenius Equation Calculator Two Temperatures.
From study.com
Using the Arrhenius Equation Chemistry Arrhenius Equation Calculator Two Temperatures The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. This activation energy calculator (also called the arrhenius equation calculator can help you calculate the minimum energy required for a chemical. Arrhenius Equation Calculator Two Temperatures.
From slideplayer.com
Chemical Chapter ppt download Arrhenius Equation Calculator Two Temperatures Arrhenius activation energy calculator for two temperatures. The arrhenius equation can be used to compare the rate constants (k) of a. Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). This article will explore the arrhenius. It provides insights into how varying temperatures affect the speed of chemical reactions. The arrhenius equation. Arrhenius Equation Calculator Two Temperatures.
From www.slideserve.com
PPT temperaturedependence of reaction rate Arrhenius equation Arrhenius Equation Calculator Two Temperatures The arrhenius equation can be used to compare the rate constants (k) of a. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based. The arrhenius equation is a formula that describes how the rate of a reaction varied based on temperature, or the rate constant. This activation energy calculator (also called the. Arrhenius Equation Calculator Two Temperatures.
From www.coursehero.com
[Solved] Alternate Form of the Arrhenius Equation At two temperatures Arrhenius Equation Calculator Two Temperatures Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). It provides insights into how varying temperatures affect the speed of chemical reactions. The arrhenius equation can be. Arrhenius Equation Calculator Two Temperatures.
From www.chemistrylearner.com
Arrhenius Equation (Plot) Definition, Form, Variables, and Constants Arrhenius Equation Calculator Two Temperatures It provides insights into how varying temperatures affect the speed of chemical reactions. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: Arrhenius activation energy calculator for two temperatures. What is the arrhenius equation for two temperatures? This activation energy calculator is helpful for. Arrhenius Equation Calculator Two Temperatures.
From calculatorey.com
Arrhenius Equation Calculator 2 Temperatures Calculatorey Arrhenius Equation Calculator Two Temperatures It provides insights into how varying temperatures affect the speed of chemical reactions. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: Arrhenius activation energy calculator for two temperatures. This activation energy calculator (also called the arrhenius equation calculator can help you calculate the. Arrhenius Equation Calculator Two Temperatures.
From atwocab.blogspot.com
Arrhenius equation Increasing temperature increases the rate reaction Arrhenius Equation Calculator Two Temperatures Users input the rate constants k1 and k2 and temperatures t1 and t2 (celsius, kelvin, or fahrenheit). This activation energy calculator is helpful for users to determine the activation energy ea using the arrhenius equation for two different temperatures. Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate. Arrhenius Equation Calculator Two Temperatures.
From www.slideserve.com
PPT Section 14.5 Activation Energy and Temperature PowerPoint Arrhenius Equation Calculator Two Temperatures Below is an arrhenius equation calculator that allows you to input two different temperatures and calculate the ratio of the rate constants at those temperatures: The arrhenius equation can be used to compare the rate constants (k) of a. It provides insights into how varying temperatures affect the speed of chemical reactions. Arrhenius activation energy calculator for two temperatures. This. Arrhenius Equation Calculator Two Temperatures.