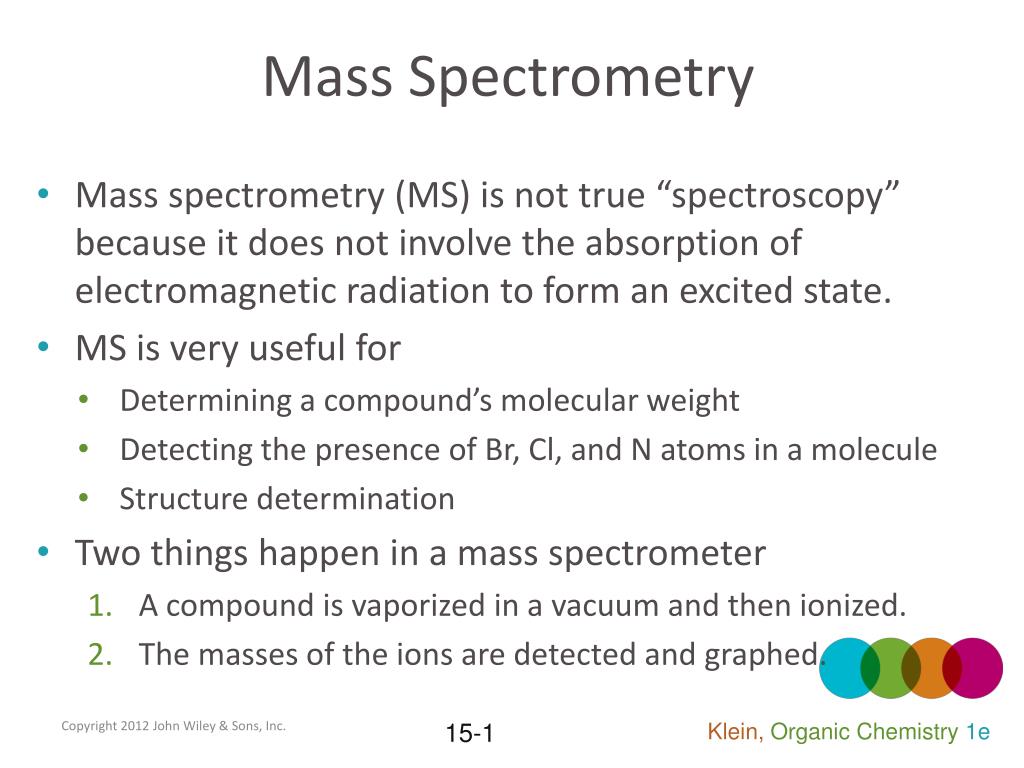
Difference Between Mass Spectrometer And Spectroscopy . Mass spectroscopy and mass spectrometry are distinctively different from each other. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Spectroscopy is simply the theoretical. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Mass spectrometry is a method that typically uses an.
from www.slideserve.com
Mass spectroscopy and mass spectrometry are distinctively different from each other. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Spectroscopy is simply the theoretical. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Mass spectrometry is a method that typically uses an. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry.
PPT Mass Spectrometry PowerPoint Presentation, free download ID5877617
Difference Between Mass Spectrometer And Spectroscopy Mass spectrometry is a method that typically uses an. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Mass spectroscopy and mass spectrometry are distinctively different from each other. Mass spectrometry is a method that typically uses an. Spectroscopy is simply the theoretical. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world.
From www.slideshare.net
Atomic Spectroscopy Basic Principles and Instruments Difference Between Mass Spectrometer And Spectroscopy The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Spectroscopy is simply the theoretical. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and.. Difference Between Mass Spectrometer And Spectroscopy.
From www.slideserve.com
PPT Molecular Mass Spectroscopy PowerPoint Presentation, free Difference Between Mass Spectrometer And Spectroscopy In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Mass spectrometry is a method. Difference Between Mass Spectrometer And Spectroscopy.
From www.youtube.com
Difference between spectroscopy and spectrometry Spectroscopy Difference Between Mass Spectrometer And Spectroscopy The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. In order to measure the characteristics of individual. Difference Between Mass Spectrometer And Spectroscopy.
From www.differencebetween.com
Difference Between Gas Chromatography and Mass Spectrometry Compare Difference Between Mass Spectrometer And Spectroscopy Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. In order to measure the characteristics of individual molecules, a mass. Difference Between Mass Spectrometer And Spectroscopy.
From www.slideserve.com
PPT Mass Spectroscopy PowerPoint Presentation, free download ID1117185 Difference Between Mass Spectrometer And Spectroscopy The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Spectroscopy is simply the. Difference Between Mass Spectrometer And Spectroscopy.
From www.slideserve.com
PPT Infrared Spectroscopy PowerPoint Presentation ID602312 Difference Between Mass Spectrometer And Spectroscopy Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Mass spectrometry is a method that typically uses an. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry,. Difference Between Mass Spectrometer And Spectroscopy.
From www.differencebetween.com
What is the Difference Between GCMS and LCMS Compare the Difference Difference Between Mass Spectrometer And Spectroscopy Spectroscopy is simply the theoretical. Mass spectroscopy and mass spectrometry are distinctively different from each other. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Spectrometry and spectroscopy are two. Difference Between Mass Spectrometer And Spectroscopy.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation, free download ID464232 Difference Between Mass Spectrometer And Spectroscopy Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world.. Difference Between Mass Spectrometer And Spectroscopy.
From spectrum-instrumentation.com
Mass Spectroscopy Spectrum Difference Between Mass Spectrometer And Spectroscopy Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Mass spectroscopy and mass spectrometry are distinctively different from each other. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Spectroscopy is simply the theoretical. In order to measure the characteristics of individual molecules, a mass spectrometer. Difference Between Mass Spectrometer And Spectroscopy.
From www.tes.com
Mass spectrometry A level Teaching Resources Difference Between Mass Spectrometer And Spectroscopy Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Some believe that the main difference is that spectroscopy typically deals with light, mass. Difference Between Mass Spectrometer And Spectroscopy.
From mavink.com
Mass Spectrometry Applications Difference Between Mass Spectrometer And Spectroscopy Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. The mass spectrometer, nmr spectrometer and. Difference Between Mass Spectrometer And Spectroscopy.
From mungfali.com
Spectrometer Vs Spectrophotometer Difference Between Mass Spectrometer And Spectroscopy Mass spectroscopy and mass spectrometry are distinctively different from each other. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Probably. Difference Between Mass Spectrometer And Spectroscopy.
From www.en.silicann.com
The difference between spectroscope, spectrometer and spectrophotometer Difference Between Mass Spectrometer And Spectroscopy Mass spectrometry is a method that typically uses an. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. The. Difference Between Mass Spectrometer And Spectroscopy.
From www.en.silicann.com
The difference between spectroscope, spectrometer and spectrophotometer Difference Between Mass Spectrometer And Spectroscopy Mass spectroscopy and mass spectrometry are distinctively different from each other. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does. Difference Between Mass Spectrometer And Spectroscopy.
From znanio.ru
14Motion of a charged particle in the field Difference Between Mass Spectrometer And Spectroscopy Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Mass spectroscopy and mass spectrometry are distinctively different from each other. Spectroscopy is simply the. Difference Between Mass Spectrometer And Spectroscopy.
From chemistrymadesimple.net
How Does A Mass Spectrometer Work? Chemistry Made Simple Difference Between Mass Spectrometer And Spectroscopy Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Spectroscopy is simply the theoretical. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Mass spectrometry is a method that typically uses an. Mass spectroscopy and mass spectrometry. Difference Between Mass Spectrometer And Spectroscopy.
From www.pinterest.fr
Spectrophotometer vs Spectrofluorometer Tabular Form Conservation Difference Between Mass Spectrometer And Spectroscopy Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Mass spectrometry is a method that typically uses an. Mass spectroscopy and mass spectrometry are distinctively different from each other. Some believe that the main difference. Difference Between Mass Spectrometer And Spectroscopy.
From www.slideserve.com
PPT Proteomics & Mass Spectrometry PowerPoint Presentation, free Difference Between Mass Spectrometer And Spectroscopy The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Mass spectrometry is a method that typically uses an. Spectrometry and spectroscopy are two closely related techniques used in. Difference Between Mass Spectrometer And Spectroscopy.
From wisc.pb.unizin.org
Isotopes, Atomic Mass, and Mass Spectrometry (M2Q3) UWMadison Difference Between Mass Spectrometer And Spectroscopy The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Spectroscopy is simply the. Difference Between Mass Spectrometer And Spectroscopy.
From www.en.silicann.com
The difference between spectroscope, spectrometer and spectrophotometer Difference Between Mass Spectrometer And Spectroscopy The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Spectrometry and spectroscopy are two closely. Difference Between Mass Spectrometer And Spectroscopy.
From www.en.silicann.com
The difference between spectroscope, spectrometer and spectrophotometer Difference Between Mass Spectrometer And Spectroscopy Mass spectrometry is a method that typically uses an. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Spectroscopy is simply the theoretical. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility. Difference Between Mass Spectrometer And Spectroscopy.
From classfulllinseeds.z21.web.core.windows.net
Mass Spectrometer Diagram And Explanation Difference Between Mass Spectrometer And Spectroscopy Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Mass spectrometry is a method that typically uses an. Mass spectroscopy and mass spectrometry are distinctively different from each other. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. The. Difference Between Mass Spectrometer And Spectroscopy.
From www.youtube.com
Difference between spectroscopy and spectrometry YouTube Difference Between Mass Spectrometer And Spectroscopy Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. The mass spectrometer, nmr spectrometer and the. Difference Between Mass Spectrometer And Spectroscopy.
From datespeck.com
Mass Spectrometry Fundamentals & Principles Datespeck Difference Between Mass Spectrometer And Spectroscopy In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Spectroscopy is simply the theoretical. Mass spectroscopy and mass spectrometry are distinctively different from each other. Mass spectrometry is a method that typically uses an. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. The mass spectrometer, nmr. Difference Between Mass Spectrometer And Spectroscopy.
From www.bronkhorst.com
Mass Spectrometry and Mass Flow Control; A closer ion them Bronkhorst Difference Between Mass Spectrometer And Spectroscopy Mass spectrometry is a method that typically uses an. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of. Difference Between Mass Spectrometer And Spectroscopy.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation, free download ID5877617 Difference Between Mass Spectrometer And Spectroscopy Mass spectroscopy and mass spectrometry are distinctively different from each other. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to. Difference Between Mass Spectrometer And Spectroscopy.
From www.creative-proteomics.com
Types of Mass Spectrometer Creative Proteomics Difference Between Mass Spectrometer And Spectroscopy Spectroscopy is simply the theoretical. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Mass spectroscopy and mass spectrometry are distinctively different from each other. The mass spectrometer, nmr spectrometer and the optical spectrometer are. Difference Between Mass Spectrometer And Spectroscopy.
From www.quora.com
What is the difference between mass spectroscopy and mass spectrometry Difference Between Mass Spectrometer And Spectroscopy Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Mass spectrometry is a method that typically uses an. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the. Difference Between Mass Spectrometer And Spectroscopy.
From 2hinst.com
Mass Spectrometry Basics for the Absolute Novice Difference Between Mass Spectrometer And Spectroscopy Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Spectroscopy is simply the theoretical. Probably one of the. Difference Between Mass Spectrometer And Spectroscopy.
From www.youtube.com
Mass spectrometryMass spectroscopyMass spectrometerPrinciple Difference Between Mass Spectrometer And Spectroscopy In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Some believe that the main difference is that spectroscopy typically deals with light, mass spectrometry does not. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Mass spectroscopy and mass spectrometry are distinctively different from each. Difference Between Mass Spectrometer And Spectroscopy.
From www.compoundchem.com
Mass Spectrometry and Interpreting Mass Spectra Compound Interest Difference Between Mass Spectrometer And Spectroscopy The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Mass spectrometry is a method that typically uses an. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry,. Difference Between Mass Spectrometer And Spectroscopy.
From www.slideserve.com
PPT Chromatography and Spectroscopy PowerPoint Presentation, free Difference Between Mass Spectrometer And Spectroscopy Mass spectrometry is a method that typically uses an. Examples of spectrometry include mass spectrometry, rutherford scattering spectrometry, ion mobility spectrometry, and. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Spectroscopy is simply the theoretical. Mass spectroscopy and mass spectrometry are distinctively different from each other. The. Difference Between Mass Spectrometer And Spectroscopy.
From www.youtube.com
Spectroscopy Vs Spectrometry_Difference between Spectroscopy and Difference Between Mass Spectrometer And Spectroscopy Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Mass spectroscopy and mass spectrometry are distinctively different from each other. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Mass spectrometry is a method that typically uses an. Spectroscopy is simply the theoretical. Spectrometry and. Difference Between Mass Spectrometer And Spectroscopy.
From schematicfixgrunwald.z19.web.core.windows.net
Mass Spectrometer Diagram And Explanation Difference Between Mass Spectrometer And Spectroscopy Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. The mass spectrometer, nmr spectrometer and the optical spectrometer are the three most common types of spectrometers found in research labs around the world. Mass spectroscopy and mass spectrometry are distinctively different from each other. Examples of spectrometry include. Difference Between Mass Spectrometer And Spectroscopy.
From thesciencenotes.com
components of mass spectrometry Archives The Science Notes Difference Between Mass Spectrometer And Spectroscopy Mass spectrometry is a method that typically uses an. Spectrometry and spectroscopy are two closely related techniques used in the field of analytical chemistry to study the interaction of matter. Mass spectroscopy and mass spectrometry are distinctively different from each other. Probably one of the most widely recognized examples of a ‘spectrometry’ method is mass spectrometry. Some believe that the. Difference Between Mass Spectrometer And Spectroscopy.