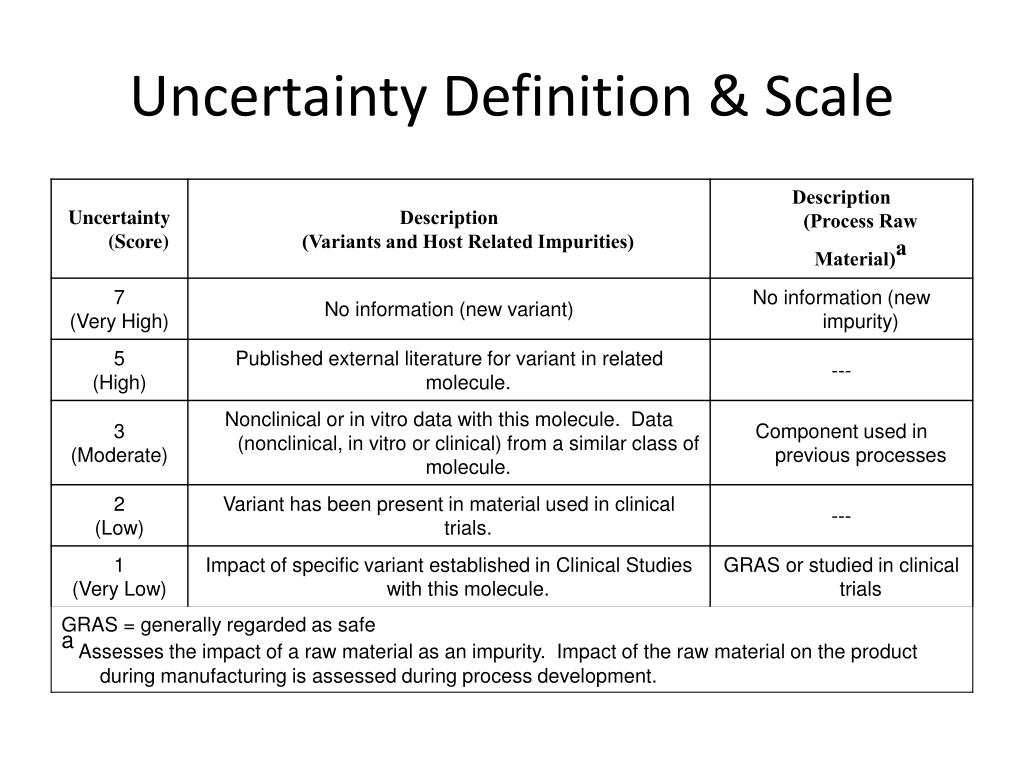
Gram Scale Uncertainty . Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. This means its mass lies between 6.722 and. One way to do this is to report the result of. This means its mass lies between.
from www.slideserve.com
This means its mass lies between 6.722 and. Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. One way to do this is to report the result of. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. This means its mass lies between. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant.
PPT Quality by Design PowerPoint Presentation, free download ID1604681
Gram Scale Uncertainty We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. This means its mass lies between. This means its mass lies between 6.722 and. One way to do this is to report the result of. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual.
From www.researchgate.net
Gramscale reaction and resolution of THN enantiomers a Gramscale Gram Scale Uncertainty Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. Chemists describe the estimated degree of. Gram Scale Uncertainty.
From www.slideserve.com
PPT Quality by Design PowerPoint Presentation, free download ID1604681 Gram Scale Uncertainty This means its mass lies between. Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. This means its mass lies between 6.722 and. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. By definition, 1 foot is exactly 12 inches, 1 inch. Gram Scale Uncertainty.
From warreninstitute.org
Calculate PERCENT UNCERTAINTY Essential Guide Gram Scale Uncertainty This means its mass lies between 6.722 and. This means its mass lies between. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. One way to do this is to report the. Gram Scale Uncertainty.
From www.researchgate.net
Uncertainty Avoidance Scale Items, Factor Loadings, and Reliability Gram Scale Uncertainty Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. This means its mass lies between. This means its mass lies between 6.722 and. One. Gram Scale Uncertainty.
From www.researchgate.net
Gramscale production of 2i and 4a by crystallization via cascade Gram Scale Uncertainty By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. If we. Gram Scale Uncertainty.
From www.scribd.com
Uncertainty Scales Gram Scale Uncertainty Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. This means its mass lies between. Was important to arrive at an internationally. Gram Scale Uncertainty.
From www.savemyexams.com
Determining Uncertainties from Graphs DP IB Physics SL Revision Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. This means its mass lies between 6.722 and. Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. One way to do this is to report the result of. If we weigh the quarter. Gram Scale Uncertainty.
From www.researchgate.net
Gramscale resolution and concise synthesis of the chiral Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. One way to do this is to report the result of. Was important to arrive at an internationally accepted procedure for expressing measurement. Gram Scale Uncertainty.
From www.researchgate.net
Classification of uncertainty types for available data and the Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report. Gram Scale Uncertainty.
From epgeoscience.com
Dealing with scale and uncertainty in the geosciences Gram Scale Uncertainty Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. This means its mass lies between. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly. Gram Scale Uncertainty.
From www.wasyresearch.com
Measurement uncertainty estimation Spreadsheets method Gram Scale Uncertainty Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. This means its mass lies between. One way to do this is to. Gram Scale Uncertainty.
From www.exceldemy.com
How to Calculate Uncertainty in Excel (3 Effective Ways) Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. Was important. Gram Scale Uncertainty.
From www.youtube.com
Calculating uncertainties 1 YouTube Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. This means its mass lies between. One way to do this is to report the result of. We must take the uncertainty in our. Gram Scale Uncertainty.
From www.slideserve.com
PPT Measurement, Uncertainty and Significant Figures PowerPoint Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. This means its mass lies between 6.722 and. One way to do this is to report the result of. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. If. Gram Scale Uncertainty.
From courses.lumenlearning.com
Measurement Uncertainty Boundless Chemistry Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. One way to do this is to report the result of. If we. Gram Scale Uncertainty.
From www.researchgate.net
Gramscale reaction with a continuousflow setup and recycling of the Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. One way to do this is to report the result of. Chemists describe the estimated degree of error in a. Gram Scale Uncertainty.
From www.youtube.com
Calculating the Average and Uncertainty in the Average YouTube Gram Scale Uncertainty One way to do this is to report the result of. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. By definition,. Gram Scale Uncertainty.
From www.muelaner.com
Calculating an Uncertainty Budget for a Measurement Dr Jody Muelaner Gram Scale Uncertainty Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. This means its mass lies between. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is. Gram Scale Uncertainty.
From www.researchgate.net
Gramscale reactions and synthetic applications a Gramscale reaction Gram Scale Uncertainty By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful. Gram Scale Uncertainty.
From www.researchgate.net
Gram matrices of quantum channels via quantum Fisher information with Gram Scale Uncertainty Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and. Gram Scale Uncertainty.
From www.researchgate.net
The scale uncertainty band of σ NNLO (gg → H) at the lHC as a function Gram Scale Uncertainty This means its mass lies between. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. This means its mass lies between 6.722 and. One way to do this is to report the result of. We must take the uncertainty in our measurements into account to avoid misrepresenting the. Gram Scale Uncertainty.
From www.youtube.com
Measurement Uncertainty IB Physics YouTube Gram Scale Uncertainty Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. This means its mass lies between 6.722 and. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Was important to arrive at. Gram Scale Uncertainty.
From www.researchgate.net
Gramscale reactions and synthetic applications a Gramscale reaction Gram Scale Uncertainty This means its mass lies between 6.722 and. One way to do this is to report the result of. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. This means its mass lies between. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1. Gram Scale Uncertainty.
From studylib.net
Chemistry Unit 1 Scale Reading, Uncertainty and Significant Figures Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful. Gram Scale Uncertainty.
From www.youtube.com
15.03 How to find the uncertainty in readings & measurements YouTube Gram Scale Uncertainty Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. One way to do this is to report the result of. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. By definition,. Gram Scale Uncertainty.
From www.wasyresearch.com
Measurement uncertainty estimation Spreadsheets method Gram Scale Uncertainty We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. This means its mass lies between 6.722 and. This means its mass lies between. Was important to arrive at an internationally. Gram Scale Uncertainty.
From courses.lumenlearning.com
Measurement Uncertainty, Accuracy, and Precision CHEM 1305 Gram Scale Uncertainty This means its mass lies between 6.722 and. This means its mass lies between. One way to do this is to report the result of. Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is. Gram Scale Uncertainty.
From www.amazon.co.uk
CGOLDENWALL TDA2 Series Analytical Balance Scale Electric Balance Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. One way to do this is to report the result of. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Was important to arrive at an internationally accepted procedure for expressing. Gram Scale Uncertainty.
From www.researchgate.net
Gramscale reaction and derivatizations Download Scientific Diagram Gram Scale Uncertainty Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. This means its mass lies between 6.722 and. Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. This means its mass lies between. One. Gram Scale Uncertainty.
From pressbooks.bccampus.ca
2.3 Measurement Uncertainty, Accuracy, and Precision CHEM 1114 Gram Scale Uncertainty Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram is exactly 0.001 kilogram. If we weigh the. Gram Scale Uncertainty.
From www.researchgate.net
Gramscale reaction and S/C evaluation Download Scientific Diagram Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. This means its mass lies between. We must take the uncertainty in our measurements into account to avoid misrepresenting the uncertainty in calculated results. By definition, 1 foot is exactly 12 inches, 1 inch is exactly 2.54 centimeters, and 1 gram. Gram Scale Uncertainty.
From www.youtube.com
Online General Chemistry Chapter 1.6 Measurement Uncertainty, Accuracy Gram Scale Uncertainty This means its mass lies between 6.722 and. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement,. Gram Scale Uncertainty.
From www.the-mad-scientist.net
Uncertainty in Measurement The!Mad!Scientist! Gram Scale Uncertainty If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. We must take the uncertainty in our measurements into account to avoid misrepresenting. Gram Scale Uncertainty.
From www.youtube.com
Averages and Uncertainty Calculations YouTube Gram Scale Uncertainty This means its mass lies between 6.722 and. Was important to arrive at an internationally accepted procedure for expressing measurement uncertainty and for combining individual. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. If we weigh the quarter on a more sensitive balance, we may find that its mass. Gram Scale Uncertainty.
From www.researchgate.net
1 Scale of uncertainty in reserves estimates Download Scientific Diagram Gram Scale Uncertainty One way to do this is to report the result of. If we weigh the quarter on a more sensitive balance, we may find that its mass is 6.723 g. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. We must. Gram Scale Uncertainty.