Sugars Are Hydrophobic . However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Hydrophobes are nonpolar molecules and. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Yet there are features in these. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule.
from www.semanticscholar.org
In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Yet there are features in these. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Hydrophobes are nonpolar molecules and.
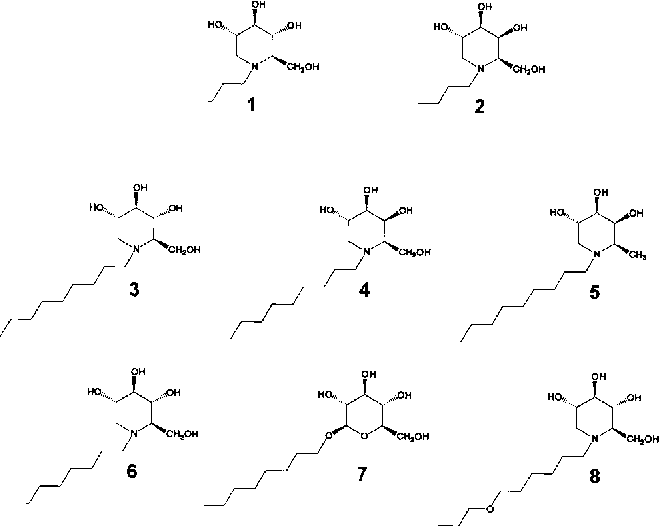
Figure 1 from Membrane disruption and cytotoxicity of hydrophobic N
Sugars Are Hydrophobic Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Yet there are features in these. Hydrophobes are nonpolar molecules and. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as
From www.chegg.com
Solved Choose which substances are hydrophobic and which are Sugars Are Hydrophobic However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps). Sugars Are Hydrophobic.
From sicklizard.deviantart.com
Hydrophobic by sicklizard on DeviantArt Sugars Are Hydrophobic However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Yet there are features in these. In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Sugars are highly water soluble, full of free. Sugars Are Hydrophobic.
From www.studocu.com
Sugar Concentration WHAT IS SUGAR Sugar is a natural ingredient that Sugars Are Hydrophobic However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Yet there are features in these. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. Carbohydrates can be. Sugars Are Hydrophobic.
From slideplayer.com
Lipid Metabolism Metabolism of dietary lipids. ppt download Sugars Are Hydrophobic Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. Hydrophobes are nonpolar molecules and. Yet there are features in these. However, because they are hydrophilic, they allow water molecules to intercalate between. Sugars Are Hydrophobic.
From www.researchgate.net
Induction model based on the sequential expression of responsive genes Sugars Are Hydrophobic Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. In food common examples of hydrophilic. Sugars Are Hydrophobic.
From courses.lumenlearning.com
Components and Structure OpenStax Biology 2e Sugars Are Hydrophobic In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Sugars have the general chemical formula ch 2 o and can be. Sugars Are Hydrophobic.
From www.academia.edu
(PDF) Membrane disruption and cytotoxicity of hydrophobic Nalkylated Sugars Are Hydrophobic Yet there are features in these. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Hydrophobes are nonpolar molecules and. In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. However, because they are. Sugars Are Hydrophobic.
From www.biolinscientific.com
Hydrophobic surfaces How hydrophobic coatings are used and studied? Sugars Are Hydrophobic Yet there are features in these. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Probably most pertinent. Sugars Are Hydrophobic.
From www.youtube.com
My first hydrophobic crush in the sizziling water with glitter💧// Soft Sugars Are Hydrophobic Hydrophobes are nonpolar molecules and. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Probably most pertinent to. Sugars Are Hydrophobic.
From www.chegg.com
Solved Hydrophilic or Hydrophobic Choose which substances Sugars Are Hydrophobic Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Yet there are features in these. Hydrophobes are nonpolar molecules and. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Sugars have the general chemical formula ch 2 o and can be joined. Sugars Are Hydrophobic.
From www.chegg.com
Solved Drag the labels to their appropriate locations in the Sugars Are Hydrophobic Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Hydrophobes are nonpolar molecules and. In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make. Sugars Are Hydrophobic.
From www.doubtnut.com
Glucose and all naturally occurring sugars are Dsugars. Sugars Are Hydrophobic Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Hydrophobes. Sugars Are Hydrophobic.
From www.semanticscholar.org
Figure 1 from Membrane disruption and cytotoxicity of hydrophobic N Sugars Are Hydrophobic Hydrophobes are nonpolar molecules and. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic. Sugars Are Hydrophobic.
From www.researchgate.net
(PDF) Simultaneous Analysis of Hydrophobic Atractylenolides, Atractylon Sugars Are Hydrophobic Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. Yet there are features in these. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in. Sugars Are Hydrophobic.
From twitter.com
The Hydrophobic (the_hydrophobic) / Twitter Sugars Are Hydrophobic Hydrophobes are nonpolar molecules and. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Yet there are features in these. In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Sugars. Sugars Are Hydrophobic.
From www.researchgate.net
(PDF) Simultaneous Analysis of Hydrophobic Atractylenolides, Atractylon Sugars Are Hydrophobic Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Hydrophobes are nonpolar molecules and. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents.. Sugars Are Hydrophobic.
From www.indiamart.com
White Hydrophobic Powder Integral Waterproofing Chemical at Rs 130 in Sugars Are Hydrophobic Yet there are features in these. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of. Sugars Are Hydrophobic.
From www.youtube.com
Hydrophobic Giant Disc 💜🤌🏻 hydrophobic gymchalkasmr hydrophobicasmr Sugars Are Hydrophobic Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Hydrophobes are nonpolar molecules and. Yet. Sugars Are Hydrophobic.
From indianavsera.weebly.com
Hydrophobic amino acids with nonpolar side chains indianavsera Sugars Are Hydrophobic Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Hydrophobes are nonpolar molecules and. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as. Sugars Are Hydrophobic.
From www.slideserve.com
PPT Chapter 3. Water— The Elixir of Life! PowerPoint Presentation Sugars Are Hydrophobic Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. Yet there are features in these. Hydrophobes are nonpolar molecules and. Probably most pertinent to this review will be what. Sugars Are Hydrophobic.
From www.researchgate.net
Sugars consumption by B. subtilis ATCC 6633 growing in CW using the Sugars Are Hydrophobic However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Yet there are features in these. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where. Sugars Are Hydrophobic.
From www.semanticscholar.org
Figure 3 from Simultaneous Analysis of Hydrophobic Atractylenolides Sugars Are Hydrophobic Hydrophobes are nonpolar molecules and. Yet there are features in these. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Carbohydrates. Sugars Are Hydrophobic.
From www.dutchbros.com
Protein Coffee Amp up your coffee! A good source of protein, high in Sugars Are Hydrophobic Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. In food common examples. Sugars Are Hydrophobic.
From www.youtube.com
MNEMONICS ON HYDROPHOBIC AND HYDROPHOBIC COMPOUNDS Sir Melvin Buracho Sugars Are Hydrophobic However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. Hydrophobes are nonpolar molecules and. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in. Sugars Are Hydrophobic.
From www.jstage.jst.go.jp
The Hydrophobic Characteristics of Sugars Sugars Are Hydrophobic Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Hydrophobes are nonpolar molecules and. In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Sugars have the general chemical formula ch. Sugars Are Hydrophobic.
From www.chegg.com
Solved Which of the following is hydrophobic? O sugars O Sugars Are Hydrophobic However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they. Sugars Are Hydrophobic.
From www.teachersuperstore.com.au
Hydrophobic Substances National Geographic Educational Resources and Sugars Are Hydrophobic However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Yet there are features in these. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of. Sugars Are Hydrophobic.
From alfiansolihin.blogspot.com
Label The Components Of A Phospholipid File 0303 Lipid Bilayer With Sugars Are Hydrophobic Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Hydrophobes are nonpolar. Sugars Are Hydrophobic.
From h-o-m-e.org
The Polar Properties of Hydrophobic Molecules Sugars Are Hydrophobic Hydrophobes are nonpolar molecules and. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Carbohydrates can be. Sugars Are Hydrophobic.
From www.sidapharm.gr
Hydrophobic IOLs Sidapharm Ophthalmic Products Sugars Are Hydrophobic Yet there are features in these. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Sugars have the general chemical formula ch 2 o and can be joined. Sugars Are Hydrophobic.
From www.researchgate.net
ZBTB7A ZF13 forms basespecific contacts. (A) Overall structure of the Sugars Are Hydrophobic Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Hydrophobes are nonpolar molecules and. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as. Sugars Are Hydrophobic.
From www.myxxgirl.com
Solved Phospholipids Form The Main Fabric Of The Plasma M Chegg Com Sugars Are Hydrophobic Yet there are features in these. Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the molecule. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. However, because they are hydrophilic, they allow water molecules to intercalate between them,. Sugars Are Hydrophobic.
From www.chegg.com
Solved A critical feature of the plasma membrane is that it Sugars Are Hydrophobic In food common examples of hydrophilic molecules in food are sugars (they have oxygen group which make them polar and they tend to be asymmetric) but also salt or. Sugars have the general chemical formula ch 2 o and can be joined together almost infinitely for storage. Probably most pertinent to this review will be what are called amphiphilic glycopolymers. Sugars Are Hydrophobic.
From surfguppy.com
What is Hydrophobic Sugars Are Hydrophobic Hydrophobes are nonpolar molecules and. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. Probably most pertinent to this review will be what are called amphiphilic glycopolymers (agps) where hydrophilic polysaccharides, or sugars,. In food common. Sugars Are Hydrophobic.
From www.european-coatings.com
Hydrophobic and biobased News and insights for the European coatings Sugars Are Hydrophobic Yet there are features in these. Sugars are highly water soluble, full of free hydroxyl groups, and not soluble in organic solvents. However, because they are hydrophilic, they allow water molecules to intercalate between them, and cannot pack as Carbohydrates can be represented by the stoichiometric formula (ch 2 o) n, where n is the number of carbons in the. Sugars Are Hydrophobic.