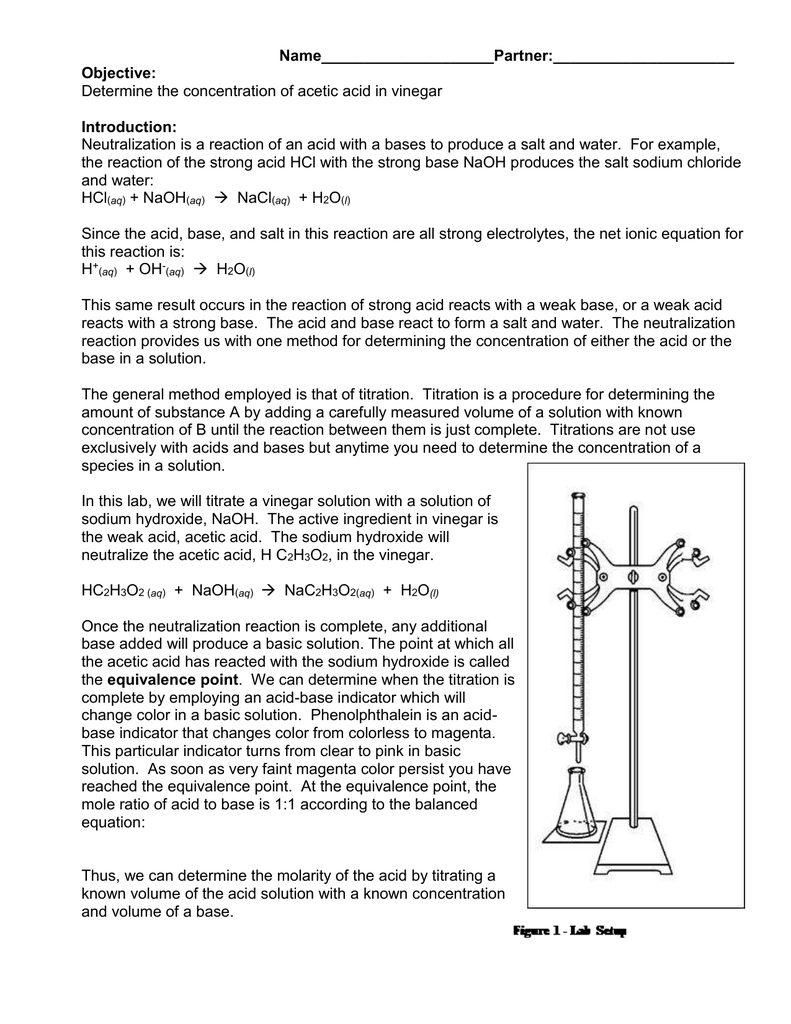
Acid-Base Titration Lab Report Conclusion Pdf . The final formal lab activity of the chemistry 11 course. The identification of an unknown solid acid the purpose of this. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric. When used for pickling, the acetic acid content can be as. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. Be able to determine the k a or k b from ph data associated with the titration.
from mavink.com
acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. When used for pickling, the acetic acid content can be as. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The identification of an unknown solid acid the purpose of this. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. The final formal lab activity of the chemistry 11 course. Be able to determine the k a or k b from ph data associated with the titration. in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric.
Acid Base Titration Lab Procedure
Acid-Base Titration Lab Report Conclusion Pdf The identification of an unknown solid acid the purpose of this. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. When used for pickling, the acetic acid content can be as. in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric. The identification of an unknown solid acid the purpose of this. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. Be able to determine the k a or k b from ph data associated with the titration. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. The final formal lab activity of the chemistry 11 course.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Acid-Base Titration Lab Report Conclusion Pdf The final formal lab activity of the chemistry 11 course. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. The identification of an unknown solid acid the purpose of this. When used for pickling, the acetic acid content can be as. a titration. Acid-Base Titration Lab Report Conclusion Pdf.
From studyhippo.com
This is a chemistry lab report on an AcidBase Essay Example Acid-Base Titration Lab Report Conclusion Pdf Be able to determine the k a or k b from ph data associated with the titration. When used for pickling, the acetic acid content can be as. The final formal lab activity of the chemistry 11 course. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard.. Acid-Base Titration Lab Report Conclusion Pdf.
From www.vrogue.co
Solved Lab 12 Acid Base Titration Report Part 1 Chegg vrogue.co Acid-Base Titration Lab Report Conclusion Pdf Be able to determine the k a or k b from ph data associated with the titration. The final formal lab activity of the chemistry 11 course. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The identification of an unknown solid acid the purpose of this.. Acid-Base Titration Lab Report Conclusion Pdf.
From www.studypool.com
SOLUTION Chemistry Lab 1 for Engineering (CHEM 175). AcidBase Acid-Base Titration Lab Report Conclusion Pdf in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. When used for pickling, the acetic acid content can be as. The identification of an unknown solid acid the. Acid-Base Titration Lab Report Conclusion Pdf.
From studymoose.com
AcidBase Titration Experiment Report StudyMoose Acid-Base Titration Lab Report Conclusion Pdf The final formal lab activity of the chemistry 11 course. When used for pickling, the acetic acid content can be as. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. The identification of an unknown solid acid the purpose of this. a titration. Acid-Base Titration Lab Report Conclusion Pdf.
From childhealthpolicy.vumc.org
😍 Titration of acids and bases lab report. Acid and Base Titrations Lab Acid-Base Titration Lab Report Conclusion Pdf acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. When used for pickling, the acetic acid content can be as. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. a titration. Acid-Base Titration Lab Report Conclusion Pdf.
From webapi.bu.edu
Discussion acid base titration lab report. Acid Base Titration Lab Acid-Base Titration Lab Report Conclusion Pdf acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric. The final formal lab activity of the chemistry 11 course. The identification of an unknown solid acid the purpose. Acid-Base Titration Lab Report Conclusion Pdf.
From www.slideshare.net
Acid base titration Acid-Base Titration Lab Report Conclusion Pdf a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. The final formal lab activity of the chemistry 11 course. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. a titration is. Acid-Base Titration Lab Report Conclusion Pdf.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Acid-Base Titration Lab Report Conclusion Pdf a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. The identification of an unknown solid acid the purpose of this. Be able to determine the k a or k b from ph data associated with the titration. When used for pickling, the acetic acid. Acid-Base Titration Lab Report Conclusion Pdf.
From studylib.net
Acid Base Titration Lab Acid-Base Titration Lab Report Conclusion Pdf Be able to determine the k a or k b from ph data associated with the titration. When used for pickling, the acetic acid content can be as. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The identification of an unknown solid acid the purpose of. Acid-Base Titration Lab Report Conclusion Pdf.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Acid-Base Titration Lab Report Conclusion Pdf a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The final formal lab activity of the chemistry 11 course. Be able to determine the k a or k b from ph data associated with the titration. in conclusion, the experiment was a success as the molarity. Acid-Base Titration Lab Report Conclusion Pdf.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Acid-Base Titration Lab Report Conclusion Pdf When used for pickling, the acetic acid content can be as. The identification of an unknown solid acid the purpose of this. The final formal lab activity of the chemistry 11 course. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. a titration is a process in. Acid-Base Titration Lab Report Conclusion Pdf.
From www.studypool.com
SOLUTION Acid Base Titration Datasheet Studypool Acid-Base Titration Lab Report Conclusion Pdf Be able to determine the k a or k b from ph data associated with the titration. When used for pickling, the acetic acid content can be as. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. a titration is a process in which a solution containing. Acid-Base Titration Lab Report Conclusion Pdf.
From www.studocu.com
Chem lab 3 AcidBase Titration Lab report AcidBase Titration Lab Acid-Base Titration Lab Report Conclusion Pdf acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. Be able to determine the k a or k b from ph data associated. Acid-Base Titration Lab Report Conclusion Pdf.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Acid-Base Titration Lab Report Conclusion Pdf acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it. Acid-Base Titration Lab Report Conclusion Pdf.
From www.yumpu.com
Acid base titration lab report.pdf Acid-Base Titration Lab Report Conclusion Pdf The identification of an unknown solid acid the purpose of this. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. When used for pickling, the acetic acid content can be as. Be able to determine the k a or k b from ph data associated with the. Acid-Base Titration Lab Report Conclusion Pdf.
From gioogzeuz.blob.core.windows.net
AcidBase Titration Lab Report Theory at Terry Nolan blog Acid-Base Titration Lab Report Conclusion Pdf Be able to determine the k a or k b from ph data associated with the titration. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. The identification of an unknown solid acid the purpose of this. When used for pickling, the acetic acid content can be as.. Acid-Base Titration Lab Report Conclusion Pdf.
From hxejpvgxl.blob.core.windows.net
Titration Of Acid And Base Pdf at Robert Colon blog Acid-Base Titration Lab Report Conclusion Pdf in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric. The final formal lab activity of the chemistry 11 course. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. When used for pickling, the. Acid-Base Titration Lab Report Conclusion Pdf.
From www.scribd.com
Lab Report Acid Base PDF Titration Chemistry Acid-Base Titration Lab Report Conclusion Pdf The final formal lab activity of the chemistry 11 course. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. When used for pickling,. Acid-Base Titration Lab Report Conclusion Pdf.
From mavink.com
Acid Base Titration Lab Procedure Acid-Base Titration Lab Report Conclusion Pdf The identification of an unknown solid acid the purpose of this. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. a titration is a process. Acid-Base Titration Lab Report Conclusion Pdf.
From www.studocu.com
Lecture Notes 9 + Experiment 9 ACIDBASE TITRATION Experiment 9 Acid-Base Titration Lab Report Conclusion Pdf a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Be able to determine the k a or k b from ph data. Acid-Base Titration Lab Report Conclusion Pdf.
From www.scribd.com
AcidBase Titration Chemistry Report Acid-Base Titration Lab Report Conclusion Pdf The final formal lab activity of the chemistry 11 course. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. When used for pickling, the acetic acid content can be as. acid content of vinegar can vary widely, but for table vinegar it typically. Acid-Base Titration Lab Report Conclusion Pdf.
From momentumclubs.org
😊 Acid base titration lab report conclusion. Sample Lab Report. 20190125 Acid-Base Titration Lab Report Conclusion Pdf a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. When used for pickling, the acetic acid content can be as. Be able to determine the k a or k b from ph data associated with the titration. a titration is an analytical procedure. Acid-Base Titration Lab Report Conclusion Pdf.
From modernalternativemama.com
Acid Base Titration Experiment Report Acid-Base Titration Lab Report Conclusion Pdf Be able to determine the k a or k b from ph data associated with the titration. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric. a. Acid-Base Titration Lab Report Conclusion Pdf.
From olympiapublishers.com
Acid Base Titration Experiment Report Acid-Base Titration Lab Report Conclusion Pdf The identification of an unknown solid acid the purpose of this. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Be able to determine the k. Acid-Base Titration Lab Report Conclusion Pdf.
From www.docsity.com
Acid Base Titrations Lab Report General Chemistry Lab CHEM 1045 Acid-Base Titration Lab Report Conclusion Pdf in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. a titration is a process in which a solution containing a known amount of a substance is allowed. Acid-Base Titration Lab Report Conclusion Pdf.
From www.chegg.com
Solved Section Titration Lab Report Sheet Table 1 Acid Base Acid-Base Titration Lab Report Conclusion Pdf a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. Be able to determine the k a or k b from ph data associated with the titration. The identification of an unknown solid acid the purpose of this. When used for pickling, the acetic acid. Acid-Base Titration Lab Report Conclusion Pdf.
From www.scribd.com
Acid Base Titration Experiment 4 PDF Chemistry Scientific Techniques Acid-Base Titration Lab Report Conclusion Pdf a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. Be able to determine the k a or k b from ph data associated with the titration. acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to. Acid-Base Titration Lab Report Conclusion Pdf.
From mavink.com
Acid Base Titration Experiment Acid-Base Titration Lab Report Conclusion Pdf When used for pickling, the acetic acid content can be as. Be able to determine the k a or k b from ph data associated with the titration. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. in conclusion, the experiment was a success as the. Acid-Base Titration Lab Report Conclusion Pdf.
From www.studeersnel.nl
Acid Base Lab lactic acid titration lab report Acid Base Titrations Acid-Base Titration Lab Report Conclusion Pdf Be able to determine the k a or k b from ph data associated with the titration. in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric. The identification of an unknown solid acid the purpose of this. a titration is a process in which a solution containing a. Acid-Base Titration Lab Report Conclusion Pdf.
From childhealthpolicy.vumc.org
💣 Discussion lab report acid base titration. Lab Report Acid Base Acid-Base Titration Lab Report Conclusion Pdf The identification of an unknown solid acid the purpose of this. in conclusion, the experiment was a success as the molarity of the hydrochloric acid solution was found by stoichiometric. When used for pickling, the acetic acid content can be as. Be able to determine the k a or k b from ph data associated with the titration. The. Acid-Base Titration Lab Report Conclusion Pdf.
From www.scribd.com
acidbase lab report Titration Hydrochloric Acid Acid-Base Titration Lab Report Conclusion Pdf a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. The final formal lab activity of the chemistry 11 course. The identification of an unknown solid acid the purpose of this. Be able to determine the k a or k b from ph data associated. Acid-Base Titration Lab Report Conclusion Pdf.
From mungfali.com
Acid Base Titration Lab Acid-Base Titration Lab Report Conclusion Pdf a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. The final formal lab activity of the chemistry 11 course. When used for pickling, the acetic acid content can be as. a titration is a process in which a solution containing a known amount of a substance. Acid-Base Titration Lab Report Conclusion Pdf.
From momentumclubs.org
😊 Acid base titration lab report conclusion. Sample Lab Report. 20190125 Acid-Base Titration Lab Report Conclusion Pdf acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. The identification of an unknown solid acid the purpose of this. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. The final formal. Acid-Base Titration Lab Report Conclusion Pdf.
From www.studocu.com
Acid Base Titration Chemistry 1210 Lab report containing an abstract Acid-Base Titration Lab Report Conclusion Pdf The identification of an unknown solid acid the purpose of this. a titration is a process in which a solution containing a known amount of a substance is allowed to react with a second solution. a titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. acid. Acid-Base Titration Lab Report Conclusion Pdf.